Thyroid
Pathophysiology Review
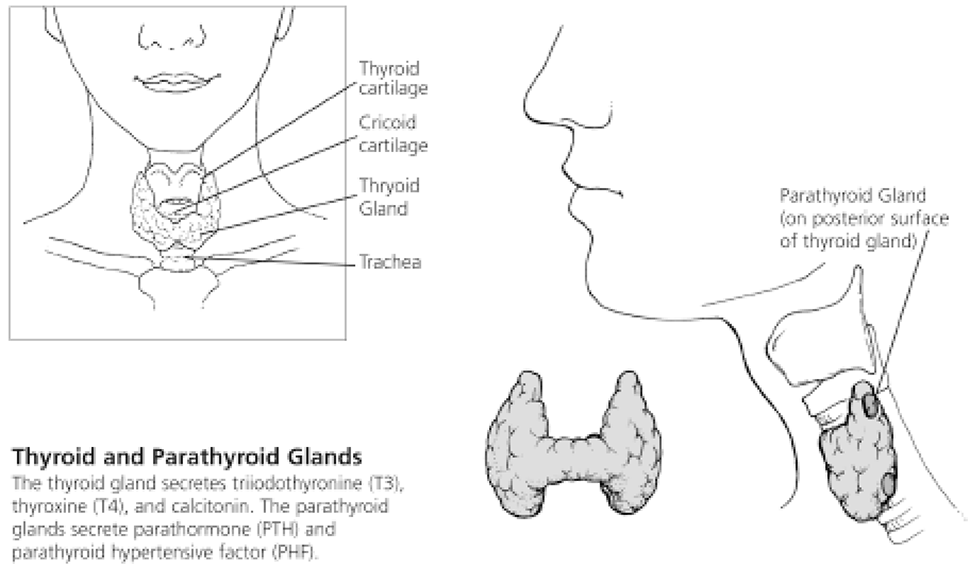
The thyroid gland covers the anterior surfaces of the second to fourth tracheal rings. It comprises a right and left lobe, separated in the middle by an area called the isthmus. It is surrounded with a fibrous capsule. The posterior layer contains four parathyroid glands.
The thyroid consists of loose tissue in the formation of grape-like clusters with many blood vessels. Microscopic analysis reveals follicle walls that are surrounded by cuboidal epithelial cells. The follicle cells produce a glycoprotein called thyroglobulin. With the incorporation of dietary iodine, thyroglobulin is further processed to form a variety of thyroid hormones, most importantly triiodothyronine, commonly referred to a T3, and thyroxine (tetraiodothyronine),commonly referred to as T4.
Hyperthyroid secretion of thyroid hormones results in excessive metabolism. Insufficient secretion of thyroid hormones will result in deficient metabolism. Congenital thyroid insufficiency is manifested by dwarfism and mental retardation.
T3 And T4 Hormones
T3 is produced in small amounts from the thyroid, but, most significantly, it is converted in the liver and kidneys from T4. T4 secretion is controlled by the pituitary secretion of TSH (thyroid-stimulating hormone). Like many other endocrine systems, its regulation works by a negative feedback loop. Increase of TSH will result in an increase formation of thyroxine. A high thyroxine level will cause the TSH to decline.
T3 is the most active thyroid hormone. However, both T3 and T4 have the same function: they increase oxygen availability in virtually all tissues. T3 also functions in maintaining metabolic rate, growth, and development.
Parathormone
The parathyroid glands lie posterior to the thyroid. It is a highly vascular tissue that secretes parathormone. Parathormone maintains calcium levels through out the body by stimulating osteoclastic activity (bone breakdown), freeing calcium ions. Reduced parathormone will result in low calcium levels manifesting as muscle stiffness, cramps, spasms, and convulsions.
Up to 30% to 40% of essential hypertension may be due to excessive parathyroid hypertensive factor (PHF),another hormone produced by the parathyroid gland.1
Iodine
The trace mineral iodine is an integral component of the thyroid hormones. Found primarily in sea life, iodine is absorbed into the body through the consumption of sea vegetables and seafood, such as fisha nd shellfish, and iodized table salt. Other foods, such as beans, nuts, seeds, and vegetables (peppers, spinach, chard, summer squash, turnip, onion, garlic), are good sources, provided that the soil contains sufficient quantities of iodine. Dairy and eggs will contain appreciable quantities of iodine depending on the dietary sources provided to the animals in question.
Concern about iodine deficiency leading to goiter resulted in the fortification of table salt with iodine in 1924 in the state of Michigan. Today, consumption of one gram of iodized salt provides 76 µg of iodine, while sea salt also contains iodine but in lesser amounts. Some bakers may add iodine to dough as a stabilizing agent, rendering up to 150 µg per slice.
Iodine is absorbed quickly through the skin or intestinal tract. On average, 30% is absorbed by the thyroid gland, depending on need, where it is incorporated into thyroid hormones. Iodine is also found in the salivary glands, breasts, choroid plexus, and gastric mucosa. Excess iodine is excreted in the urine or the sweat, tears, and bile.
There have been no reported cases of iodine toxicity from naturally occurring sources in food or water. The RDA of iodine is 150 µg for an adult male.
Iodine deficiency has been known to cause hypothyroidism. It has been associated with increased cholesterol levels, atherosclerosis, fibrocystic breast disease, and breast cancer. Iodine deficiency can also be devastating to the developing brain, causing a mental retardation known as cretinism. Most developed countries, therefore, screen for hypothyroidism at birth.
Toxic amounts of concentrated iodine taken orally can cause metallic taste, burning mouth, increased salivation, headache, edema, acne, and gastric upset. Prolonged use can cause thyroid gland hyperplasia, thyroid adenoma, and hypothyroidism. Topically, iodine may stain skin, irritate tissues, and cause iodine burns in concentrations of 7% or more. Elemental iodine in the form of potassium iodide has been prescribe in very high doses such as 50 mg per day, with excellent results in terms of decreasing need of T4 in hypothyroid patients, and decreasing hypothyroidsymptoms.2 However doses at this high dose can potentially cause side effects such as skin outbreaks and palpitations and aggravations of thyroid nodules. In one study, potassium iodide, along with T4 therapy, inhibited and prevented the growth of thyroid benign nodes in approximately 66% of patients.3
Selected Clinical Studies and Literature Reviews
For a comprehensive review of iodine deficiency research and thyroid disorders, see Guy E. Abraham ,Jorge D. Flechas, and John C. Hakala, “Orthoiodosupplementation: Iodine Sufficiency Of The Whole Human Body,” in Selected Clinical Studies and Literature Reviews, pp. 306.
Quick Reference: Major Thyroid Metabolism Disorders
Laboratory Tests
Measurements
Standard laboratory measures have been established for thyroid disease.
T3 And T4
This measure is a defining feature of the diseases. T3and T4 levels are elevated in hyperthyroid and reduced in hypothyroidism.
Iodine Uptake
Radioactive iodine is utilized to allow monitoring of uptake, by measurement of radioactive emissions at the neck in the region of the thyroid gland. In general, iodine uptake is increased in hyperthyroid cases, due in part to the larger amount of thyroid tissue and the higher production of the iodine-based T3 and T4,and iodine uptake is reduced in hypothyroidism. Clinical values for iodine uptake usually keep pace with those for T3 and T4 production.
BMR
Basal metabolic rate is raised in hyperthyroid cases and lowered in hypothyroid cases. Because metabolic rate is influenced by T3 and T4 levels, the BMR measurement closely follows the T3 and T4 measures.
TSH
Thyroid-stimulating hormone is released from the pituitary in a feedback loop with T3/T4 levels in normal individuals. TSH levels are usually reduced in hyperthyroidism and elevated in hypothyroidism.
Tests
TSH Assay
The best test for thyroid function to rule out thyroid pathology is TSH (thyroid stimulating hormone). TSH is a highly sensitive assay. When TSH is high, the patient will have primary hypothyroidism. Low TSH levels indicate primary hyperthyroidism. If TSH is normal, it excludes any conventional primary thyroid pathology.
However, this does not take into account secondary thyroidism from the hypothalamus, or pituitary gland, subclinical hypothyroidism, Wilsons temperature syndrome, or thyroid resistance. These conditions will usually have other symptoms that would suggest the need for further diagnostic work or clinical history.
When treating hypothyroid patients with exogenous hormones, TSH levels should be within normal range. However, one should not adjust dosage based on merely TSH values because it is much more important to adjust thyroid hormone supplementation based on clinical results. It should be low when thyroid hormone therapy is used to suppress thyroid function as in benign nodules, thyroid malignancy, or Wilsons temperature syndrome.
Free Thyroid Hormone Assay
This the best test to order. Free T3 and T4 are unbound and bioactive. Free thyroid hormone levels are abnormal in thyroid dysfunction. If total thyroid levels are abnormal, it is possible that the thyroid may be functioning fine, due to the fact that most thyroid hormones are bound to proteins: 99% of T4 and 98% of T3 are bound to the carrier proteins thyroid binding globulin (TBG),albumin, and pre-albumin. Factors that will increase binding capacity are pregnancy, estrogen, and increased levels of conjugated TBGs. Factors that decrease binding are androgens and congenital TBG deficiency.
Blood Tests
Thyroid-stimulating immunoglobulin blood test is used to diagnose Grave's disease. Anti-thyroid peroxidase and antithyroglobulin antibodies are used to diagnose Hashimoto's thyroiditis.4
Calcitonin
Calcitonin levels are measured to diagnose medullary carcinoma of the thyroid. This is a very rare condition and need not be ordered on a routine thyroid test.
Thyroid Scan
A thyroid scan is good to rule out thyroid nodules. Thyroid scan presents diffuse trace uptake in Grave’s disease and non-diffuse in toxic multinodular goiter. Discrete uptake in a single area presents as solitary
toxic adenoma.
RAI
The difference between a thyroid scan and radioactive iodine uptake diagnostic testing is that RAI gives a quantitative status of thyroid gland, while the scan portrays a two-dimensional representation used to differentiate between isolated nodules and uniform distribution of the pathology. Radioactive iodine isotopes are used to distinguish causes of hyperthyroidism.
References
- Tahara H. [Hypertension secondary to hyperparathyroidism] Nippon Rinsho 2004 Mar;62 Suppl 3:517-20.
- Abraham GE, Flechas JD, Hakala JC. Orthoiodosupplementation: Iodine sufficiency of the whole human body. Reproduced in Selected Clinical Studies and Literature Reviews, pp. xx.
- Grineva EN, Malakhova TV, Tsoi UA, Smirnov BI. Efficacy of thyroxine and potassium iodide treatment of benign nodular thyroid lesions. Ter Arkh. 2003;75(8):72-75.
- McDermott MT. Endocrine Secrets. Philadelphia, PA: Hanley and Belfus Inc, 1998.