Abstract
Objective: This case study reports on the novel combination of three treatments for cognitive decline in a 79-year-old male: therapeutic plasma exchange (TPE), peptides, and human umbilical cord tissue mesenchymal stem cells and exosomes (UCT-MSC-EXs).
Methods: Cognitive function was evaluated with the Central Nervous System-Vital Signs (CNS-VS) test. Biological age and telomere lengths were measured at baseline, 9 months, and 16 months using TruAge. The patient received four rounds of TPE (5% albumin exchange) administered using the Amicus Separator. He was also treated with UCT-MSC-EXs manufactured by Vitti Labs (Liberty, MO, USA). In addition, he was given two peptides, Semax and Epitalon, to address cognitive decline.
Results: After 1 year of treatment using TPE with 5% albumin, UCT-MSC-EXs, Semax and Epitalon, the patient’s biological age was reduced by 7.9 years (from 75.93 to 68.03), and his telomere length increased from 6.45 to 6.59 kb. As the CNS-VS test documented, his cognitive function improved from April 2022 to December 2023.
Conclusion: To our knowledge, this is the first case study to report on reducing biological age, increasing telomere length, and improving cognition using this unique combination of therapies in a patient with mild to moderate cognitive decline.
Introduction
Cognitive decline may occur for a variety of reasons, including the accumulation of senescent cells,1 excessive oxidative stress,2 mitochondrial dysfunction,3 loss of apoptosis,4 environmental toxins5,6 depletion of stem cells,7 telomere shortening,8 inefficient cell communication,9 epigenetic alterations10 and neuroinflammation.11 Once cognitive decline starts, its path is usually a downward spiral. According to the World Health Organization, more than 55 million people are affected with dementia, and nearly 10 million new cases are diagnosed each year. In 2019, dementia cost the global economy 1.3 trillion US dollars. It is estimated that up to 107 million people worldwide will be affected by some form of dementia by 2050.12 Currently, there are no therapies that can reverse dementia. This paper reports on an innovative combination of treatments, therapeutic plasma exchange (TPE), peptides, and human umbilical cord tissue mesenchymal stem cells and exosomes (UCT-MSC-EXs), which improved cognitive function and biological age in a 79-year-old male.
Therapeutic Plasma Exchange
Therapeutic plasma exchange with albumin replacement has been shown to improve Alzheimer’s disease (AD). Over 100 years ago, physicians experimented with TPE’s potential to increase lifespan when the first recorded plasma exchange procedure was performed on animals. The term “apheresis,” often used to describe TPE, came from that experiment.13 In 1993, The Journal of Clinical Apheresis published a paper detailing the use of TPE as either a first- or second-line therapy to be used with other modalities on disorders such as Raynaud’s disease, systemic lupus erythematosus, coagulation factor inhibitors, familial hypercholesterolemia, acute Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, myasthenia gravis, cryoglobulinemia, Goodpasture’s syndrome, and thrombotic thrombocytopenic purpura.14 Present indications for use include pathologies such as multiple sclerosis (MS), AD, and other neurological, hematological, rheumatological, and nephrological conditions.15–17 TPE is gaining ground for its potential to reduce oxidative stress, inflammation, senescent cells, and improve immune function.18
The treatment protocol for TPE consists of removing and retaining a considerable volume of plasma, approximately 1 to 1.5 plasma volume,17 and returning the necessary cellular components via a colloid or combination colloid/crystallization solution of either fresh frozen plasma (FFP) or albumin.19 There are two types of TPE devices: centrifugation and filter-based. Most TPE treatments in the United States use the centrifugation method, which separates blood components into layers of red blood cells, plasma, platelets, granulocytes, monocytes, and lymphocytes according to density. Those cellular components are returned to the patient with FFP or albumin. The removed plasma is discarded. Filtration devices use a membrane with pores optimally sized for only protein to pass through.15
Mechanistic actions of TPE include removing toxic agents, cytokines, and abnormal inflammatory proteins20 such as amyloid beta (Aβ) proteins21; reducing autoantibody concentration19; and replacing any deficient cellular components.17 With immune-mediated disorders, TPE can alter the T-helper type 1 and T-helper type 2 cell ratio by increasing the predominance ratio from Th-2 to Th-1, thereby boosting humoral immune response and modulating cellular immunity.19 Studies show that after TPE treatment, natural killer cells and CD8 T cells increase, reducing inflammatory macrophages and enhancing adaptive immunity.20 Using the colloid solution albumin to replace volume has many therapeutic effects, including reducing inflammation, accelerating healing, supporting immunomodulation, increasing antioxidant load, and maintaining intravascular colloid osmotic pressure.20,22
Peptides
The peptides we used in this case report, Epitalon and Semax, have been studied in Russia for over 30 years, including in clinical trials.23 Both peptides are approved for human use in Russia.24 Epitalon is a 4-amino acid pluripotent peptide and regulator of the brain, pineal glands, and retina.25 Epitalon increases brain-derived neurotrophic factor (BDNF) and cyclic-AMP responsive element binding protein 1 by regulating melatonin synthesis and circadian gene expression. Melatonin decreases with age and is essential for preventing neurological decline.26 Epitalon weighs 0.39 kDa, which means it is small enough to interact with DNA and act as a regulatory factor.27,28 Studies show that Epitalon can epigenetically regulate gene expression of neuronally differentiated cells and protein synthesis in human stem cells and increase the synthesis of several markers of neurogenic differentiation.29
Semax is a seven amino acid peptide and a synthetic fragment of adrenocorticotropic hormone (ACTH) known in clinical practice for its nootropic and neuroprotective properties and protease stability.30 In 2001, Semax was introduced in Russia as 1% nasal drops for Parkinson’s disease, encephalopathy, cerebral vascular accidents, and other vascular deficient pathologies of the brain.24 A 2018 clinical trial with 110 patients with ischemic stroke found that Semax increased BDNF.31 Intranasal administration of Semax at 16 mg/kg in healthy adults improved decision-making, work site performance, mental acuity, short-term memory, and attention span, with results lasting up to 24 hours.32
Exosomes
Exosomes are released through the plasma membrane and originate from the endosomal pathway.33 Exosomes are a class of small extracellular vesicles (30–100 nm) released by most cells.34 They are found in body fluids such as saliva, urine, semen, milk, and blood.35,36 They can carry various cargo, including microRNAs (miRNAs), short noncoding RNA, proteins, DNA, messenger RNA (mRNA), nucleic acids and lipids.34 Exosomes are involved with angiogenesis, immune responses, cell adhesion and migration, inflammatory response, metabolic adaptation, fetal development, and intercellular communication.35–38 Exosomes are small and can cross the blood–brain barrier (BBB).
In an animal model of AD, mesenchymal stem cell (MSC)-derived exosomes delivered intranasally reduced extracellular plaque, inflammation, and glial activation.39 It is speculated that exosomes may partly improve AD symptoms by protecting neurons from oxidative stress.40 Recent studies have used exosomes with various human diseases, including diseases caused by mitochondrial dysfunction.41
Mesenchymal Stem Cells
The MSCs used in this case report were derived from umbilical cord tissue. MSCs may be sourced from human umbilical cord, adipose tissue, Wharton’s jelly, or bone marrow.42 They can migrate to specific injury sites and differentiate into multiple cells and tissues, such as glial cells, neurons, or chondrogenic tissues.42,43 MSCs release BDNF, which stimulates neural progenitor cells and magnifies their anti-apoptotic and antioxidant effects. They also inhibit neural cell necrosis and induce the proliferation of astrocytes.42 In general, MSCs do not cross the BBB. However, specific injuries such as traumatic brain injury, stroke, brain tumor, or aging are believed to compromise the efficiency of BBB protection, thereby allowing MSCs access to cross the BBB and deliver their protective effects.44–47
MSCs promote neurogenesis48 by releasing exosomes, producing numerous benefits such as reducing glial activation and brain Aβ levels and improving cognition.43 Another beneficial health impact of MSCs is enhanced mitochondrial function. In a mouse model of myocardial infarction, directly transplanted exogenous mitochondria derived from mesenchymal stem cells were better at protecting cardiac function than mitochondria derived from skin fibroblasts.49 Both MSCs and exosomes can reduce inflammation and improve oxidative stress.50,51
In a recent study, MSCs enhanced autophagy and exerted a neuroprotective effect by modulating amyloid-β clearance in an AD mouse model.52 Further, the safety and tolerability of MSCs as a treatment for AD have been shown in a phase 1 clinical trial. In the trial, nine patients with mild-to-moderate AD were injected with human umbilical cord blood-derived mesenchymal stem cells via stereotactic intraparenchymal surgical injection. The outcome produced a safe and effective route of administration without adverse effects.53 Another study used the same stereotactic method and transplanted MSCs into the brains of mice with Parkinson’s disease. Thirty-six months later, they were shown to be safe, without signs of tumor formation or other adverse side effects.54 The delivery of MSC with intravenous, intramuscular, or intranasal for the treatment of neurodegenerative disease is still debatable.
Case Presentation
The patient is a 79-year-old former business owner and stockbroker. A friend referred him to the Institute for Hormonal Balance, Orlando, FL, for health optimization. At the first appointment in April 2022, he had trouble finishing his sentences and difficulty with memory recall. He stated that his memory had been declining for about 10 years and that he had issues with short-term memory. He lived most of his life in Colorado where he had never seen a neurologist, nor had he ever been diagnosed with dementia. His short-term memory issues made him concerned that he had an early form of dementia. Given the cost of a brain MRI or PET scan, he focused on treatment rather than extra diagnostic testing.
The patient had a history of hypothyroidism, prediabetes, hyperlipidemia, hypertension, overactive bladder, sleep apnea, and narcolepsy. He was on seventeen supplements and the following medications: mirabegron (Myrbetriq) 50 mg QD for overactive bladder; hydrochlorothiazide 12.5 mg QD and losartan 50 mg QD for hypertension; levothyroxine 125 mcg QD for hypothyroidism; and armodafinil 50 mg QD for narcolepsy. On the first visit, his vital signs were: blood pressure 149/85 and pulse 80 bpm. He weighed 92.5 kg and was 170.2 cm tall.
We used the CNS-VS, a computerized neurocognitive test, to assess his cognitive function (see Methods). At baseline he scored less than a 2% range in complex attention, cognitive flexibility, and executive function. In other areas, such as the neurocognition index and verbal memory, his scores were in the 2–8 percentile range. His composite memory, psychomotor speed, reaction time, processing speed, and simple attention were in the low-average category. Out of 12 categories, he scored within the average range on only visual memory and motor speed and did not score above average in any category. Table 1 shows baseline CNS-VS test results, with treatment dates shown in Table 2. CNS-VS test results after treatment are shown in Table 3.
Table 1: Baseline cognitive function test results, April 2022.
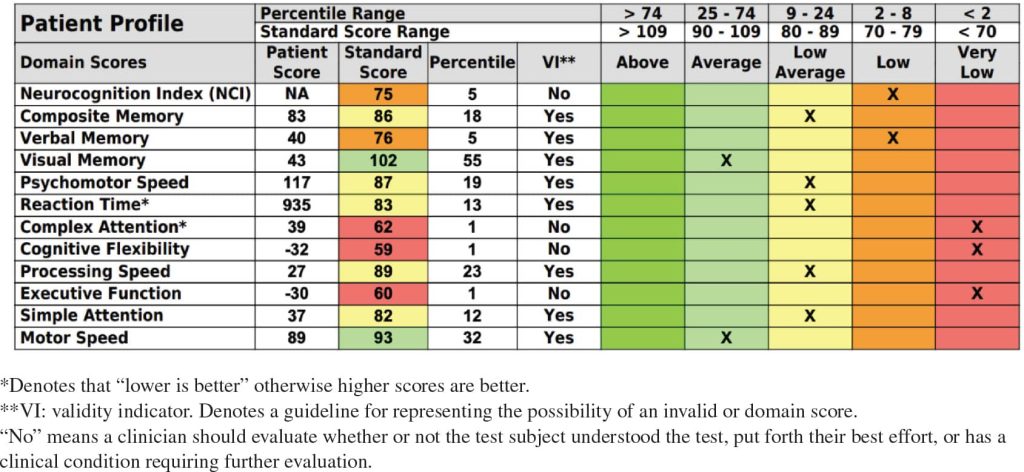
Table 2: Treatment dates.
| Date | TPE | UCT-MSC-EXs, Epitalon therapy | Semax |
|---|---|---|---|
| Aug 2022 | Semax | ||
| Sep 2022 | Semax | ||
| Oct 2022 | TPE #1 (half exchange) | Semax | |
| Nov 2022 | UCT-MSC-EXs | Semax | |
| Dec 2022 | Epitalon | Semax | |
| Jan 2023 | UCT-MSC-EXs and Epitalon | Semax | |
| Feb 2023 | Semax | ||
| Mar 2023 | Semax | ||
| Apr 2023 | TPE #2 (half exchange) | Semax | |
| May 2023 | TPE #3 (full exchange) | Semax | |
| Jun 2023 | TPE #4 (full exchange) | Epitalon | Semax |
| Jul 2023 | Semax | ||
| Aug 2023 | Semax |
TPE, therapeutic plasma exchange; UCT-MSC-EXs, umbilical cord tissue mesenchymal stem cell and exosomes.
Table 3: Treatment dates and TruAge measurements.
| Date | TPE | UCT-MSC-EXs, Epitalon therapy | Chronological age (years) | Biological age (years) | Telomere length |
|---|---|---|---|---|---|
| Apr 2022 | 79.35 | 75.93 | 6.45 kb | ||
| Oct 2022 | TPE #1 | ||||
| Nov 2022 | UCT-MSC-EXs | ||||
| Dec 2022 | Epitalon | ||||
| Jan 2023 | UCT-MSC-EXs and Epitalon | 80.12 | 73.87 | 6.45 kb | |
| Apr 2023 | TPE #2 | ||||
| May 2023 | TPE #3 | ||||
| Jun 2023 | TPE #4 | Epitalon | |||
| Aug 2023 | 80.67 | 68.3 | 6.59 kb |
Semax was given from Aug 2022 to Aug 2023.
TPE, therapeutic plasma exchange; UCT-MSC-EXs, umbilical cord tissue mesenchymal stem cell and exosomes.
The patient’s initial biological age testing by TruAge on April 30, 2022 showed an intrinsic epigenetic age of 75.93, a chronological age of 79.35, a Dunedin Pace (a rate of aging test whose optimal value is less than 1) value of 1.00, and a telomere length of 6.45 kb.
The patient consented to undergo a Food & Drug Administration (FDA)-approved TPE treatment using 5% albumin. During the consent process, he was informed that other treatments he would receive were experimental and not FDA-approved. He was told that he was not enrolled in a clinical trial and was informed of possible side effects of the treatments, including that his current condition might worsen.
Methods
We could perform only a half exchange of TPE for the first two sessions because of the patient’s request to stop due to his need to use the restroom. Beforehand, we tried having the patient wear an external catheter, but it did not fit him properly. As a result, only approximately 1300 cm3 of plasma was removed and replaced with six vials of 250 cm3 of 5% albumin per session versus the optimal 2600 cm3 full exchange. After his last two TPEs, the patient skipped breakfast so he would not have to void during future treatments. That allowed us to conduct a full exchange of TPE, removing approximately 2600 cm3 of plasma per session, with nine to ten vials of 250 cm3 of 5% albumin replacement fluid.
In addition to the four TPE sessions, he consented to the experimental use of UCT-MSC-EXs and peptide therapy to help with his cognition. The peptide Semax was taken at a daily dose of 750 mcg of nasal spray in each nostril from August 2022 until the last TruAge blood test in August 2023. The peptide Epitalon was administered subcutaneously at a dose of 5 mg for ten consecutive days in December 2022, January 2023, and June 2023. One day of intranasal UCT-MSC-EXs therapy began in November 2022, and his second 1-day treatment of intranasal UCT-MSC-EXs began in January 2023. A total of 2 ml of UCT-MSC-EXs was given intranasally with approximately ten sprays per nostril. Table 2 shows dates for each component of the treatment protocol.
Background Information For Tests, Laboratory Equipment, And Technical Considerations
Cognitive Function
Cognitive function was assessed using a computerized neurocognitive test, CNS-VS, with CNS software (CNS Vital Signs, Chapel Hill, NC). The CNS-VS assesses nine cognitive functions: memory, verbal memory, visual memory, processing speed, executive functioning, psychomotor speed, reaction time, complex attention, and cognitive flexibility. The Neurocognition Index (NCI) is calculated based on five tasks: memory, psychomotor speed, reaction time, complex attention, and cognitive flexibility. The computer report from the CNS-VS test provides subject scores, standard scores, percentiles, and assessments according to a 5-degree scale for each of the nine cognitive functions examined and the Neurocognition Index. These assessments are as follows: above average (more than 109), average (90–109), low average (80–89), low (70–79), and very low (less than 70).55
Biological Age and Telomere Length
These were evaluated using TruAge (developed by TruDiagnostic Inc., Lexington, KY). Peripheral whole blood samples were obtained using the lancet and capillary method, then mixed with lysis buffer to preserve the cells. DNA extraction was performed, and 500 ng of DNA was subjected to bisulfite conversion using the EZ DNA Methylation Kit from Zymo Research, following the manufacturer’s protocol. The bisulfite-converted DNA samples were randomly allocated to designated Illumina Infinium EPIC850k Beadchip wells. The samples were amplified, hybridized onto the array, and subsequently stained. After washing steps, the variety was imaged using the Illumina iScan SQ instrument to capture raw image intensities, enabling further analysis. The algorithms analyzed by TruAge include first- (Horvath and Hannum), second- (phenoAge, systemsAge, OMICAge, and GrimAge), and third-generation clocks (DunedinPACE). This study predicted telomere length via DNA methylation data using DNAmTL.56
Mesenchymal Stem Cells (MSCs) and Exosomes
Vitti Labs is an FDA-registered, American Association of Tissue Banks (AATB) accredited, Current Good Manufacturing Practice (CGMP) certified tissue bank. The donated tissue was bio-ethically bestowed and collected from the hospital operating room from a full-term c-section birth with a healthy mother and baby. All tissue procured has been screened by using Chagas enzyme immunoassay (EIA) test antibodies to the human immunodeficiency virus, type 1 and type 2 (anti-HIV-1 and anti-HIV-2) Plus O, Nucleic Acid Test (NAT) for HIV-1, hepatitis B surface antigen (HbsAg), hepatitis B, core total Ab, hepatitis C virus Ab (anti-HCV), cytomegalovirus (CMV) total Ab, HTLV I/II Ab, West Nile virus (WNV), ZIKA NAT, Nucleic Acid Test (NAT) for the hepatitis B virus, hepatitis C virus, and human immunodeficiency virus (HBV/HCV), syphilis, and Lyme disease. In addition, the donor was screened following the AATB standards for medical history, genetic screening, and vaccinations, including COVID-19. Once an AATB-accredited procurement agency collected the tissue, sterility was maintained throughout the custody of the tissue.
MSCs and exosomes were isolated from the umbilical cord tissue of Wharton’s jelly. They were tested using Flow Cytometry Services from RayBiotech (Corners, GA) for the following surface markers: CD20−, CD34−, CD45−, HLA-DR−, DC73+, CD90+, CD105+, CD166+. The MSCs were then expressed to release exosomes with CD9+, CD63+, and CD81+ surface markers. The exosomes were collected and purified using Tangential Flow Filtration. The number of exosomes consisted of 15 billion per milliliter. The size distribution was between 50 and 90 nm in diameter. These measurements were taken using direct Stochastic Optical Reconstruction Microscopy via nanoimager from Oxford Nanoimaging. The concentrations were measured using Tunable Resistive Pulse Sensing (TRPS) from Izon Sciences. Quality assurance was taken for sterility by testing 20% of the final lot, using USP 71 guidelines for sterility testing,57 USP 85 endotoxin testing,58 and USP 1116 for environmental testing59 following all cGMP standards and ISO 5 conditions. They were shipped in a validated shipper on dry ice and stored in a cryo-freezer at negative 80°C at the Institute for Hormonal Balance.
Therapeutic Plasma Exchange (TPE)
TPE was performed using an Amicus Separator from Fresenius Kabi. The anticoagulant chosen was anticoagulant citrate dextrose solution, Solution A. The replacement fluid was 70% of 5% albumin and 30% normal saline. Two separate intravenous (IV) catheters were inserted, one in each arm, and sometimes an ultrasound was used to locate the venous access in the patient’s arm. The patient’s weight, height, and latest hematocrit were entered into the Amicus to calculate the amount of plasma to be removed and the amount of 5% albumin to receive. We used one of his arm veins as the inlet and the other arm vein as the return line, where he received 5% albumin plus his filtered blood.
Epitalon and Semax
These peptides were obtained from an FDA-approved 503A compounding pharmacy in the United States. Semax is a nasal spray; one spray in each nostril was used daily, each containing 750 mcg. The dose of Epitalon used was 5 mg, subcutaneously for 10 consecutive days (one vial contained 50 mg of Epitalon). The dosing of Epitalon was based on a 15-year study done in Russia.60 Both peptides were kept in the refrigerator.
Results
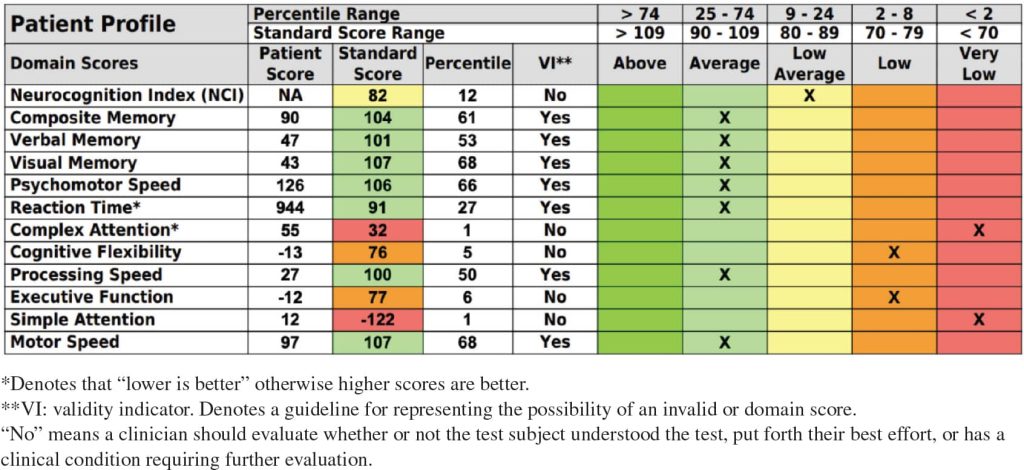
The patient was given Semax from August 2022 until August 2023. He began receiving the combination of his other therapies (TPE, UCT-MSC-EXs, and Epitalon) over 8 months, soon after starting on his initial peptide. After 1 year of treatment, the patient’s biological age was reduced by 7.9 years (from 75.93 to 68.03), and his telomere length increased from 6.45 to 6.59 kb (Table 3). After he had received the combination of all therapies (TPE, UCT-MSC-EXs, Epitalon, and Semax), the patient’s CNS-VS testing, performed in December 2023 (18 months after baseline testing), showed significant improvement in composite memory, verbal memory, reaction time, and psychomotor speed, going from 2/12 in the average category at baseline to 7/12 in the average category (Table 4).
Table 4: Post-treatment cognitive function test results, December 2023.
Discussion
TPE has been shown to improve many signs of aging-related cognitive decline. These improvements include reducing Aβ plaque build-up in the brain, enhancing language and verbal fluency, and restoring interferon responsiveness.61 One of the most well-known studies of TPE, the double-blind placebo-controlled AD management by albumin replacement (AMBAR) trial, hypothesized that withdrawing plasma might flush Aβ plaque from the brain into the plasma where it could be removed, thereby reducing AD signs and symptoms. Researchers randomly assigned 347 patients to three TPE treatment arms with different doses of albumin and intravenous immunoglobulin replacement for 1 year. The AMBAR study found that TPE with albumin replacement facilitated the binding and removal of amyloid beta protein to the cerebrospinal fluid and plasma, which was associated with improved verbal memory, language fluency, and processing speed.62–64
In another study with a subset of AMBAR trial participants, TPE with albumin replacement reduced neuroinflammation.65 TPE has also been shown to decrease the effects of environmental toxins.66,67 To reiterate, the volume of plasma removed in the first two TPE sessions was less than we intended due to the patient’s break for the restroom. Thus, only 1300 cm3 of plasma was removed versus 2600 cm3. Under ideal conditions, similar to those in the AMBAR study in which 2600–3000 cm3 of plasma was removed from the patients, his biological age may have been reduced more.
After reading Khavinson et al. (2022)25 on the possible benefit to neurogenesis of combining Epitalon with MSCs, we added Epitalon to the treatment protocol in January 2023 to be taken before and during UCT-MSC-EXs. We chose intranasal administration of UCT-MSC-EXs as we felt it delivered better efficacy than intravenous administration for crossing the BBB. To validate, intranasal delivery of MSC-derived exosomes reduced extracellular plaque, inflammation, and glial activation in a mouse model of AD.39 Another study that compared intravenous, intrathecal, and intranasal administration of exosomes found that intranasal administration was best for delivery to the brain.68 And, a first-in-human open-label interventional study in 10 neonates showed that intranasal delivery of bone marrow-derived MSCs was safe and had no adverse side effects.69
The epigenetic clock has been verified to measure biological age.70,71 We used TruAge to measure our patient’s biological age, performing the tests three times. The first test was administered in April 2022, and the last was in August 2023. Our patient’s biological age dropped by 7.63 years, whereas his chronological age went up by 1.32 years. We speculate that the reduction observed in biological age resulted from removing environmental or exogenous toxins through TPE and 5% albumin. While TPE forms part of a beneficial approach to toxic load reduction, the extent to which it was the reason in this case study is unknown, as the patient opted not to do further testing for financial reasons.
Depletion of MSCs and inefficient cell signaling can be detrimental to aging changes. We used UCT-MSC-EXs to help combat MSC depletion and exosomes to improve intercellular communication. MSCs have been shown to increase telomere length in mice,72 while Epitalon has been shown to increase telomerase activity and telomere length in human somatic cells.73 Telomeres are responsible for maintaining DNA integrity. Short telomeres have been associated with accelerated aging, AD, diabetes, heart disease, cancer, and other medical conditions.74 Dysfunctional telomeres arise from critically short telomeres, ultimately leading to replicative cellular senescence and chromosome instability: both hallmarks of aging.75
The 1.4 kb increase in the patient’s telomere length may have resulted from the combination of Epitalon and UCT-MSC-EXs, potentially improving his mitochondrial function and biological age. Again, because the patient’s first two TPE sessions were interrupted due to going to the bathroom, we could remove only 1300 cm3 rather than 2600 cm3 of plasma. Had the sessions gone as designed, his biological age may have been reduced further with the removal of 2600 cm>3 plasma from each of the four TPE sessions.
We are conducting a study with other patients at the Institute for Hormonal Balance to determine if Epitalon alone can reduce biological aging.
Conclusion
The patient reported in this paper had not previously seen a neurologist or been diagnosed with AD or another form of dementia. He declined to have a brain MRI or PET scan for financial reasons, choosing instead to focus on treatment. At the first appointment, he had trouble finishing sentences and recalling memory.
Before his third TPE, he reported that his short-term memory had improved, stating that it felt as if a “switch had turned on” in his brain. He also reported being better able to complete his thoughts during conversations and finish his daily activities without forgetting what he was supposed to do. We will continue his protocol of TPE with 5% albumin replacement, UCT-MSC-EXs, Epitalon, and Semax indefinitely. However, the future of Epitalon and Semax depends on the FDA’s September 2023 ban on 22 peptides, including Epitalon and Semax, due to safety issues associated with their use in compounding.76 In addition, we will monitor his fibrinogen level and cut back on the number of TPE sessions if it drops below the lower average level of 1.5 g/L77 to avoid the risk of excessive bleeding. We will also continue to evaluate his biological age with the TruAge testing analysis.
Although we cannot prove that this patient generated new neurons or synapses, our results using this combination of therapies (TPE with 5% albumin replacement, peptides, and UCT-MSc-EXs) are truly promising. Not only did he report positive changes, but his tests also demonstrated clinical improvement in cognitive function, a reduction in biological age by almost 8 years, and a 0.14 kb increase in telomere length. More studies using this treatment approach are needed to corroborate our findings. Still, we present them to offer hope to the patients and families of patients who are experiencing mild to moderate cognitive decline.
Competing Interests
The authors report no competing interests.
References
- Liu R-M. Aging, cellular senescence, and Alzheimer’s disease. Int J Mol Sci. 2022;23(4):1989. https://doi.org/10.3390/ijms23041989.
- Cioffi F, Adam R, Broersen K. Molecular mechanisms and genetics of oxidative stress in Alzheimer’s disease. J Alzheimers Dis. 2019;72(4):981–1017. https://doi.org/10.3233/jad-190863.
- Peng Y, Gao P, Shi L, et al. Central and peripheral metabolic defects contribute to the pathogenesis of Alzheimer’s disease: targeting mitochondria for diagnosis and prevention. Antioxid Redox Signal. 2020;32(16):1188–36. https://doi.org/10.1089/ars.2019.7763.
- Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441(7095):880–4. https://doi.org/10.1038/nature04723.
- Pamphlett R, Kum Jew S. Different populations of human locus ceruleus neurons contain heavy metals or hyperphosphorylated tau: implications for amyloid-β and tau pathology in Alzheimer‘s disease. J Alzheimers Dis. 2015;45(2):437–47. https://doi.org/10.3233/jad-142445.
- Mazzatenta A, Pokorski M, Sartucci F, et al. Volatile organic compounds (VOCs) fingerprint of Alzheimer’s disease. Respir Physiol Neurobiol. 2015;209:81–4. https://doi.org/10.1016/j.resp.2014.10.001.
- López-Otín C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell. 2013;153(6):1194–217. https://doi.org/10.1016/j.cell.2013.05.039.
- Shim H, Horner JW, Wu C-J, et al. Telomerase reverse transcriptase preserves neuron survival and cognition in Alzheimer’s disease models. Nat Aging. 2021;1(12):1162–74. https://doi.org/10.1038/s43587-021-00146-z.
- Fafián-Labora J, O’Loghlen A. Classical and nonclassical intercellular communication in senescence and ageing. Trends Cell Biol. 2020;30(8):628–39. https://doi.org/10.1016/j.tcb.2020.05.003.
- Ijomone OM, Ijomone OK, Iroegbu JD, et al. Epigenetic influence of environmentally neurotoxic metals. Neurotoxicology. 2020;81:51–65. https://doi.org/10.1016/j.neuro.2020.08.005.
- Rajesh Y, Kanneganti T-D. Innate immune cell death in neuroinflammation and Alzheimer’s disease. Cells. 2022;11(12):1885. https://doi.org/10.3390/cells11121885.
- Nichols E, Steinmetz JD, Vollset S, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health. 2022;7(2), e105–25. https://doi.org/10.1016/s2468-2667(21)00249-8.
- Abel J, Rowntree L, Turner B. Plasma removal with return of corpuscles (plasmaphaeresis). J Pharmacol Exp Ther. 1914;5:625–41.
- Strauss RG, Ciavarella D, Gilcher RO, et al. An overview of current management. J Clin Apher. 1993;8(4):189–94. https://doi.org/10.1002/jca.2920080402.
- Reeves HM, Winters JL. The mechanisms of action of plasma exchange. Br J Haematol. 2014;164(3):342–51. https://doi.org/10.1111/bjh.12629.
- Tonev D, Momchilova A. Therapeutic plasma exchange and multiple sclerosis dysregulations: focus on the removal of pathogenic circulatory factors and altering nerve growth factor and sphingosine-1-phosphate plasma levels. Curr Issues Mol Biol. 2023;45(10):7749–74. https://doi.org/10.3390/cimb45100489.
- Bobati SS. Therapeutic plasma exchange - an emerging treatment modality in patients with neurologic and non-neurologic diseases. J Clin Diagn Res. 2017;11:EC35–37. https://doi.org/10.7860/jcdr/2017/27073.10480.
- Kim D, Kiprov DD, Luellen C, et al. Old plasma dilution reduces human biological age: a clinical study. Geroscience. 2022;44(6), 2701–20. https://doi.org/10.1007/s11357-022-00645-w.
- Tonev DG, Momchilova AB. Therapeutic plasma exchange in certain immune-mediated neurological disorders: focus on a novel nanomembrane-based technology. Biomedicines. 2023;11(2):328. https://doi.org/10.3390/biomedicines11020328.
- Kiprov DD, Herskowitz A, Kim D, et al. Case report: therapeutic and immunomodulatory effects of plasmapheresis in long-haul covid. F1000Res. 2022;10:1189. https://doi.org/10.12688/f1000research.74534.2.
- Saito Y, Kobayakawa M, Sakata M, et al. P2-057: Removal of aβ oligomers from the blood using hollow fibers: a potential therapeutic system for Alzheimer’s disease. Alzheimers Dement. 2018;14(7S_Part_12):P688 https://doi.org/10.1016/j.jalz.2018.06.742.
- Boada M, López OL, Olazarán J, et al. A randomized, controlled clinical trial of plasma exchange with albumin replacement for Alzheimer’s disease: primary results of the AMBAR study. Alzheimers Dement. 2020;16(10):1412–25. https://doi.org/10.1002/alz.12137.
- Ponomareva-Stepnaya MA, Porunkevich EA, Skuin’sh AA, et al. Hormonal activity of an acth4-10 analog--a long-acting learning stimulator. Bull Exp Biol Med. 1986;101(3):255–6. https://doi.org/10.1007/bf00835912.
- Kolomin T, Shadrina M, Slominsky P, et al. A new generation of drugs: synthetic peptides based on natural regulatory peptides. Neurosci Med. 2013;4(4):223–52. https://doi.org/10.4236/nm.2013.44035.
- Khavinson V, Diomede F, Mironova E, et al. AEDG peptide (Epitalon) stimulates gene expression and protein synthesis during neurogenesis: possible epigenetic mechanism. Molecules. 2020;25(3):609. https://doi.org/10.3390/molecules25030609.
- Ilina A, Khavinson V, Linkova N, Petukhov M. Neuroepigenetic mechanisms of action of ultrashort peptides in Alzheimer’s disease. Int J Mol Sci. 2022;23(8):4259. https://doi.org/10.3390/ijms23084259.
- Kolchina N, Khavinson V, Linkova N, et al. Systematic search for structural motifs of peptide binding to double-stranded DNA. Nucleic Acids Res. 2019;47(20):10553–63. https://doi.org/10.1093/nar/gkz850.
- Ohno M, Fornerod M, Mattaj IW. Nucleocytoplasmic transport: the last 200 nanometers. Cell. 1998;92(3):327–36. https://doi.org/10.1016/s0092-8674(00)80926-5.
- Burger D, Stihle M, Sharma A, et al. Crystal structures of the human doublecortin C- and N-terminal domains in complex with specific antibodies. J Biol Chem. 2016;291(31):16292–306. https://doi.org/10.1074/jbc.m116.726547.
- Magrì A, Tabbì G, Giuffrida A, et al. Influence of the N-terminus acetylation of Semax, a synthetic analog of ACTH (4-10), on copper(II) and zinc(II) coordination and biological properties. J Inorg Biochem. 2016;164:59–69. https://doi.org/10.1016/j.jinorgbio.2016.08.013.
- Gusev EI, Martynov MY, Kostenko EV, et al. The efficacy of Semax in the tretament of patients at different stages of ischemic stroke. Zh Nevrol Psikhiatr Im SS Korsakova. 2018;118(3):61–8. https://doi.org/10.17116/jnevro20181183261-68.
- Kaplan A, Kochetova A, Nezavibathko V, et al. Synthetic acth analogue Semax displays nootropic-like activity in humans. Neurosci Res Commun. 1996;19(2):115–23. https://doi.org/10.1002/(sici)1520-6769(199609)19:23.0.co;2-b.
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. https://doi.org/10.1126/science.aau6977.
- Zhang Y, Liu Y, Liu H, Tang W. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9(1):19. https://doi.org/10.1186/s13578-019-0282-2.
- Osaid Z, Haider M, Hamoudi R, Harati R. Exosomes interactions with the blood–brain barrier: implications for cerebral disorders and therapeutics. Int J Mol Sci. 2023;24(21):15635. https://doi.org/10.3390/ijms242115635.
- Vlachakis D, Mitsis T, Nicolaides N, et al. Functions, pathophysiology and current insights of exosomal endocrinology (review). Mol Med Rep. 2020;23(1):26. https://doi.org/10.3892/mmr.2020.11664.
- Álvarez S, Contreras-Kallens P, Aguayo S, et al. Royal jelly extracellular vesicles promote wound healing by modulating underlying cellular responses. Mol Ther Nucleic Acids. 2023;31:541–52. https://doi.org/10.1016/j.omtn.2023.02.008.
- Rodeo SA. Exosomes: the new kid on the block in orthobiologics. Am J Sports Med. 2023;51(13):3363–6. https://doi.org/10.1177/03635465231207060.
- Cone AS, Yuan X, Sun L, et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer’s disease-like phenotypes in a preclinical mouse model. Theranostics. 2021;11(17):8129–42. https://doi.org/10.7150/thno.62069.
- Jiang L, Gu Y, Du Y, et al. Exosomes: diagnostic biomarkers and therapeutic delivery vehicles for cancer. Mol Pharm. 2019;16(8):3333–49. https://doi.org/10.1021/acs.molpharmaceut.9b00409.
- Li X, Han Y, Meng Y, Yin L. Small RNA-big impact: exosomal miRNAs in mitochondrial dysfunction in various diseases. RNA Biol. 2024;21(1):1–20. https://doi.org/10.1080/15476286.2023.2293343.
- Oyebode O, Tulay P. Mesenchymal stem cells applications in Alzheimer’s disease. Glob Med Genet. 2023;10(4):382–7. https://doi.org/10.1055/s-0043-1777087.
- Caputi S, Trubiani O, Sinjari B, et al. Effect of short peptides on neuronal differentiation of stem cells. Int J Immunopathol Pharmacol. 2019;33:205873841982861. https://doi.org/10.1177/2058738419828613.
- Graham N, Sharp DJ. Understanding neurodegeneration after traumatic brain injury: from mechanisms to clinical trials in dementia. J Neurol Neurosurg Psychiatry. 2019;90(11):1221–33. https://doi.org/10.1136/jnnp-2017-317557.
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008;28(38):9451–62. https://doi.org/10.1523/jneurosci.2674-08.2008.
- Arvanitis CD, Ferraro GB, Jain RK. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2019;20(1):26–41. https://doi.org/10.1038/s41568-019-0205-x.
- Erickson M, Banks W. Age-associated changes in the immune system and blood–brain barrier functions. Int J Mol Sci. 2019;20(7):1632. https://doi.org/10.3390/ijms20071632.
- Bodart-Santos V, de Carvalho LP, de Godoy M, et al. Extracellular vesicles derived from human Wharton’s jelly mesenchymal stem cells protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers. Stem Cell Res Ther. 2019;10(1):332. https://doi.org/10.1186/s13287-019-1432-5.
- Liang X, Zhang Y, Lin F, et al. Direct administration of mesenchymal stem cell-derived mitochondria improves cardiac function after infarction via ameliorating endothelial senescence. Bioeng Transl Med. 2022;8(1):e10365. https://doi.org/10.1002/btm2.10365.
- Zhang W, Wang T, Xue, et al. Research progress of extracellular vesicles and exosomes derived from mesenchymal stem cells in the treatment of oxidative stress-related diseases. Front Immunol. 2023;14:1238789. https://doi.org/10.3389/fimmu.2023.1238789.
- Farfán N, Carril J, Redel M, et al. Intranasal administration of mesenchymal stem cell secretome reduces hippocampal oxidative stress, neuroinflammation and cell death, improving the behavioral outcome following perinatal asphyxia. Int J Mol Sci. 2020;21(20):7800. https://doi.org/10.3390/ijms21207800.
- Shin J, Park H, Kim H, et al. Mesenchymal stem cells enhance autophagy and increase β-amyloid clearance in Alzheimer’s disease models. Autophagy. 2013;10(1):32–44. https://doi.org/10.4161/auto.26508.
- Kim H, Seo S, Chang J, et al. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: a phase 1 clinical trial. Alzheimer’s Dement. 2015;1(2):95–102. https://doi.org/10.1016/j.trci.2015.06.007.
- Chia Y, Anjum C, Yee H, et al. Stem cell therapy for neurodegenerative diseases: how do stem cells bypass the blood-brain barrier and home to the brain? Stem Cells Int. 2020;2020:8889061. https://doi.org/10.1155/2020/8889061.
- Gualtieri C, Johnson L. Reliability and validity of a computerized neurocognitive test battery, CNS vital signs. Arch Clin Neuropsychol. 2006;21(7):623–43. https://doi.org/10.1016/j.acn.2006.05.007.
- Lu AT, Seeboth A, Tsai P-C, et al. DNA methylation-based estimator of telomere length. Aging. 2019;11(16):5895–923. https://doi.org/10.18632/aging.102173.
- General chapters: sterility tests. U.S. Pharmacopeia; n.d. Retrieved January 31, 2024. http://ftp.uspbpep.com/v29240/usp29nf24s0_c71.html.
- General chapters: bacterial endotoxins test. Pharmacopeia; n.d. Retrieved January 31, 2024. http://www.uspbpep.com/usp29/v29240/usp29nf24s0_c85.html.
- General chapters: microbiological evaluation of clean rooms and other controlled environments. U.S. Pharmacopeia; n.d. Retrieved January 31, 2024. http://ftp.uspbpep.com/v29240/usp29nf24s0_c1116.html.
- Korkushko OV, Khavinson VK, Shatilo VB, et al. Peptide geroprotector from the pituitary gland inhibits rapid aging of elderly people: results of 15-year follow-up. Bull Exp Biol Med. 2011;151(3):366–9. https://doi.org/10.1007/s10517-011-1332-x.
- Navarro-Martínez R, Cauli O. Therapeutic plasmapheresis with albumin replacement in Alzheimer’s disease and chronic progressive multiple sclerosis: a review. Pharmaceuticals. 2020;13(2):28. https://doi.org/10.3390/ph13020028.
- Grifols. Grifols AMBAR results demonstrate a significant reduction in the progression of moderate Alzheimer’s disease. October 27, 2018. https://www.grifols.com/en/view-news/-/news/grifols-ambar-results-demonstrate-a-significant-reduction-in-the-progression-of-moderate-alzheimers-disease.
- Boada M, Anaya F, Ortiz P, et al. Efficacy and safety of plasma exchange with 5% albumin to modify cerebrospinal fluid and plasma amyloid-β concentrations and cognition outcomes in Alzheimer’s disease patients: a multicenter, randomized, controlled clinical trial. J Alzheimer’s Dis. 2017;56(1):129–43. https://doi.org/10.3233/jad-160565.
- Boada M, López OL, Olazarán J, et al. Neuropsychological, neuropsychiatric, and quality-of-life assessments in Alzheimer’s disease patients treated with plasma exchange with albumin replacement from the randomized AMBAR study. Alzheimer’s Dement. 2021;18(7):1314–24. https://doi.org/10.1002/alz.12477.
- Ortiz A, Minguet C, Gonzalo R, et al. Inflammatory biomarkers in patients undergoing therapeutic plasma exchange with albumin replacement as a treatment for Alzheimer’s disease. Alzheimer’s Dement. 2021;17(S5):e057735. https://doi.org/10.1002/alz.057735.
- King J, Kern MH, Jaar BG. Extracorporeal removal of poisons and toxins. Clin J Am Soc Nephrol. 2019;14(9):1408–15. https://doi.org/10.2215/cjn.02560319.
- Dişel N, Akpınar A, Sebe A, et al. Therapeutic plasma exchange in poisoning: 8 years’ experience of a university hospital. Am J Emerg Med. 2015;33(10):1391–5. https://doi.org/10.1016/j.ajem.2015.07.016.
- Tolomeo A, Zuccolotto G, Malvicini R, et al. Biodistribution of intratracheal, intranasal, and intravenous injections of human mesenchymal stromal cell-derived extracellular vesicles in a mouse model for drug delivery studies. Pharmaceutics. 2023;15(2):548. https://doi.org/10.3390/pharmaceutics15020548.
- Baak LM, Wagenaar N, van der Aa NE, et al. Feasibility and safety of intranasally administered mesenchymal stromal cells after perinatal arterial ischaemic stroke in the Netherlands (passion): a first-in-human, open-label intervention study. Lancet Neurol. 2022;21(6):528–36. https://doi.org/10.1016/s1474-4422(22)00117-x.
- Shireby GL, Davies JP, Francis PT, et al. Recalibrating the epigenetic clock: implications for assessing biological age in the human cortex. Brain. 2020;143(12):3763–75. https://doi.org/10.1093/brain/awaa334.
- Gensous N, Sala C, Pirazzini C, et al. A targeted epigenetic clock for the prediction of biological age. Cells. 2022;11(24):4044. https://doi.org/10.3390/cells11244044.
- Liu Q, Song S, Song L, et al. Mesenchymal stem cells alleviate aging in vitro and in vivo. Ann Transl Med. 2022;10(20):1092–2. https://doi.org/10.21037/atm-22-1206.
- Khavinson VK, Bondarev IE, Butyugov AA. Epithalon peptide induces telomerase activity and telomere elongation in human somatic cells. Bull Exp Biol Med. 2003;135(6):590–2. https://doi.org/10.1023/a:1025493705728.
- Rizvi S, Raza S, Mahdi F. Telomere length variations in aging and age-related diseases. Curr Aging Sci. 2014;7(3):161–7. https://doi.org/10.2174/1874609808666150122153151.
- Aguado J, d’Adda di Fagagna F, Wolvetang E. Telomere transcription in ageing. Ageing Res Rev. 2020;62:101115. https://doi.org/10.1016/j.arr.2020.101115.
- FDA. Safety risks associated with certain bulk drug substances nominated for use in compounding. U.S. Food & Drug Administration; December 12, 2023. Retrieved March 3, 2024. https://www.fda.gov/drugs/human-drug-compounding/safety-risks-associated-certain-bulk-drug-substances-nominated-use-compounding.
- Fish RJ, Neerman-Arbez M. Fibrinogen gene regulation. Thromb Haemost. 2012;108(09):419–26. https://doi.org/10.1160/th12-04-0273.