Abstract
The body's defense against oxidative stress is multilayered, involving a host of endogenous antioxidants and enzymes, as well as exogenous dietary factors that provide additional support. The gut microbiome's role is highlighted in its ability to produce antioxidative enzymes and metabolites, which contribute to maintaining redox balance. These antioxidant products of the gut microbiome support the structural integrity of the intestinal barrier and also play a protective role in mitochondrial health, thus influencing systemic physiological processes. The delicate interplay between oxidative processes and antioxidative defenses is known as redox homeostasis. This review discusses the critical role of redox biology in human health, exploring the essential equilibrium between oxidative stress and antioxidant mechanisms for cellular function and overall vitality. We explore the multifaceted causes of oxidative stress, from intrinsic factors like mitochondrial activity, inflammation, and immune responses to extrinsic influences such as environmental pollutants and dietary components.
Introduction
Redox Chemistry
Reduction-oxidation (redox) reactions are crucial biochemical processes that involve the transfer of electrons between molecules. These reactions are central to cellular physiology, supporting vital functions such as metabolism, cell signaling, energy production, and detoxification.1 In these processes, reducing agents (also known as antioxidants) donate electrons, while oxidizing agents accept them. Normal cellular activities generate reactive species, including reactive oxygen species (ROS) like superoxide (O2−) and hydrogen peroxide (H2O2), and reactive nitrogen species. These reactive molecules, due to their unpaired electrons, are highly potent oxidizing agents that can remove electrons from other molecules to stabilize themselves (Figure 1).
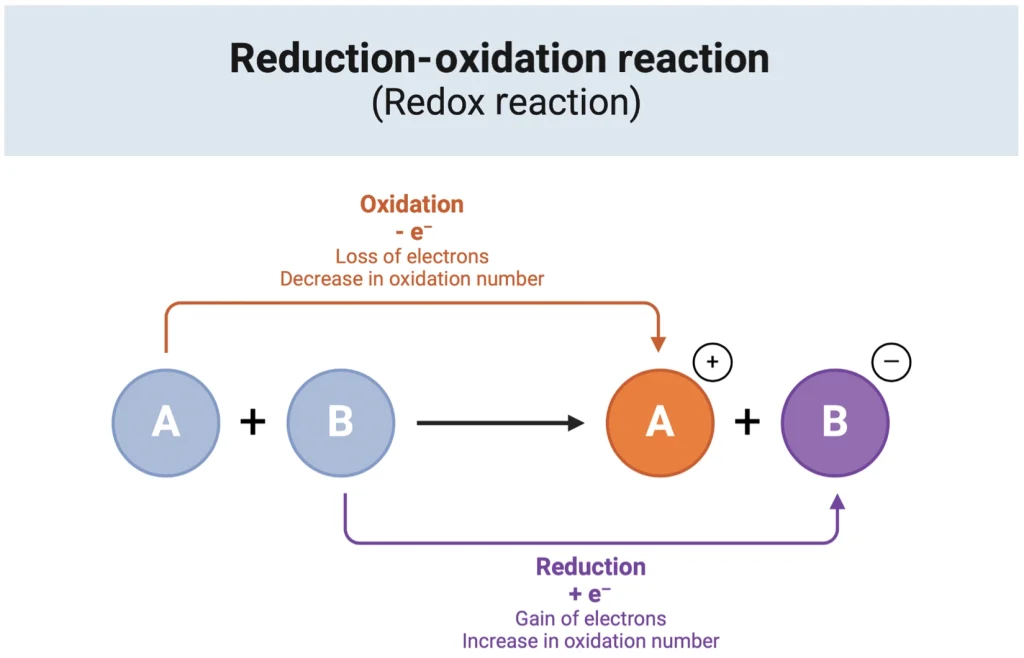
Such reactive molecules, in controlled amounts, play important roles in cell signaling, immune responses, and gene regulation. However, the accumulation of excessive reactive species can have broad damaging effects and must be counteracted by the body's antioxidant systems. An imbalance in this redox equilibrium, caused either by an overproduction of reactive species or a deficiency in antioxidant defenses, results in oxidative stress. This condition can cause extensive cellular damage and is implicated in the onset and progression of various diseases.3 Therefore, maintaining a balance between oxidants and antioxidants is essential for preserving cellular integrity and overall health, highlighting the importance of redox homeostasis in human well-being.
The redox paradox (Figure 2) highlights a fundamental contradiction within biological systems: oxidative processes are indispensable for normal cellular functionality yet can instigate cellular damage, potentially initiating or worsening a wide array of diseases. This paradox accentuates the vital importance of redox homeostasis—a meticulous equilibrium between oxidants and antioxidants essential for preserving health.
Over millennia, this delicate balance has evolved in parallel with the conflict between infectious diseases and the unintended consequences of chronic inflammation. No cellular mechanisms are 100% efficient, so free radicals and reactive oxidative species are produced. Today, further disruption of this redox balance is becoming more prevalent, mirroring and even intensifying the surge in health challenges faced by modern society.
Introduction to Redox and the Gut Microbiome
The gut microbiome, a complex community of microorganisms residing in our gastrointestinal (GI) tract, plays a pivotal role in maintaining health and disease. A growing body of research points to the importance of its influence on the body's redox balance as a key part of this role in health. This dynamic ecosystem impacts metabolic, immune, and neurological functions and plays a crucial role in both contributing to and protecting against oxidative stress. Dysbiosis, or the imbalance of gut microbial communities, can precipitate shifts in redox balance, leading to increased oxidative stress and contributing to various diseases, from metabolic disorders to inflammatory and neurodegenerative conditions.5-7 Dysbiosis is characterized by elevated availability of electron acceptors in the gut (oxidizing agents) and a high redox potential in the gut, which influences microbial growth and colonization through microbial competition for electron acceptors.8,9 Conversely, a healthy microbiome, through its production of antioxidant metabolites, modulation of immune responses, and stimulation of antioxidant response elements (AREs) can help maintain redox equilibrium, showcasing the potential of microbiome-based interventions in restoring health. The complex interplay between the gut microbiome and redox processes, pivotal for understanding disease mechanisms and therapeutic opportunities, will be explored in detail in this article. These interactions highlight the microbiome's broad role in health and disease and its significant impact on redox homeostasis, particularly in its ability to influence the gut environment's redox potential.
Health Implications of Oxidative Stress
In the current health landscape, particularly within Western societies, the disruption of redox homeostasis represents a critical health concern. Widespread oxidative stress, arising from this disruption, is linked to a range of systemic conditions and is even posited to accelerate aging, as suggested by the free radical theory of aging.10 It undermines the integrity of the gut microbiome and mucosa, resulting in heightened permeability and dysbiosis. This disturbance impacts not only GI and systemic health but also impairs the body's mechanisms for maintaining redox homeostasis and antioxidant functionality.11,12 Beyond the GI tract, oxidative stress initiates pro-inflammatory responses and disrupts immune function, contributing to a range of systemic diseases.
The rise in chronic diseases is often associated with systemic redox imbalances and exacerbated by lifestyle and environmental factors that promote oxidative stress.13,14 Oxidative stress plays a role in a growing list of conditions, including GI diseases, cancers, neurodegenerative disorders like Alzheimer's and Parkinson's, metabolic syndromes such as type 2 diabetes, and cardiovascular diseases like atherosclerosis.15,16 The widespread impact of oxidative stress on health underscores the need for a deeper understanding and the development of strategies to prevent and treat such conditions, emphasizing the importance of maintaining redox homeostasis for overall health.
Factors Influencing Redox in the Human Body
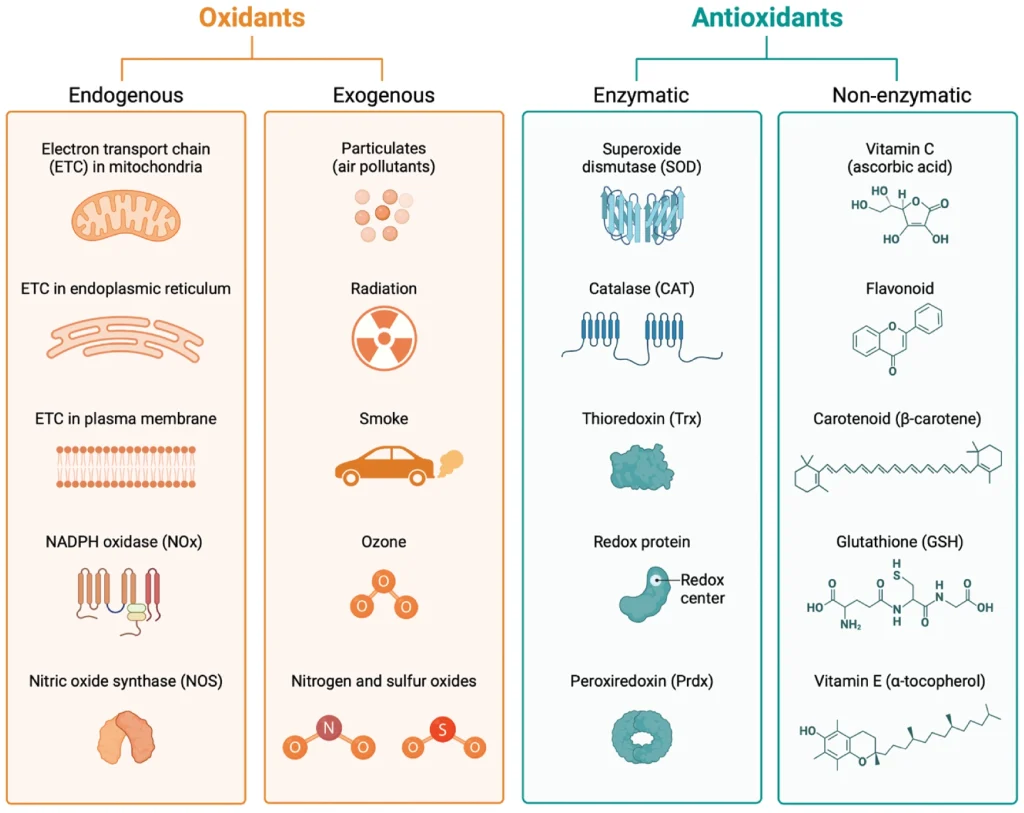
In the intricate balance of redox chemistry, antioxidants and oxidants engage in a continuous interplay critical for maintaining cellular health (Figure 3).14,16 Oxidants, generated both internally during metabolic activities and by external environmental factors, threaten cellular integrity by potentially damaging DNA, proteins, and lipids. Antioxidants counter these threats by neutralizing oxidants through electron donation, effectively preventing cellular damage.18 This neutralization process is supported by both endogenous antioxidant systems and exogenous sources obtained from dietary intake of vitamins and plant phytochemicals. Moreover, the gut microbiome plays a crucial role in this dynamic by influencing the body's redox balance through the production of metabolic by-products with antioxidant properties.19,20 This multi-component system illustrates the interconnectedness of diet, environmental exposures, and internal metabolic processes in the regulation of redox balance for the prevention of oxidative stress.
Oxidant Factors
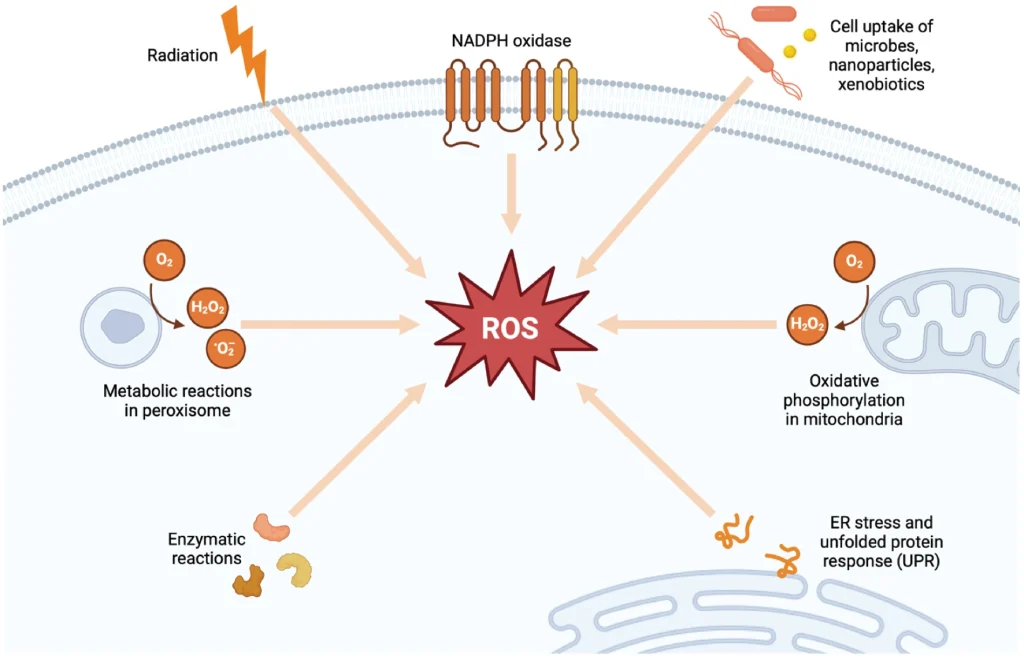
Physiological factors play a key role in the generation of free radicals and other reactive molecules within the body. Mitochondria are a primary source of ROS, where electron leakage from within the electron transport chain leads to ROS formation during the energy production process, an integral component of regular metabolic function.21 Within the mitochondrial respiratory chain, complexes I and III are the main sites for superoxide radical production—mitochondrial DNA is especially vulnerable to oxidative damage due to its close location to ROS production and limited repair mechanisms.13 The GI mucosa and immune cells also significantly contribute to free radical production, particularly in conditions of increased mucosal defense and inflammation.9,12 Inflammation and stress further amplify ROS production, with active inflammation in mucosal regions causing a swift depletion of nutrients and oxygen, which in turn increases the oxygen demand and exacerbates oxidative stress.11
Environmental Factors
External factors, including environmental pollutants like heavy metals, herbicides, and air pollution, as well as ingested oxidants found in processed foods and certain medications, further exacerbate the body's oxidative burden.22 These substances can induce cytochrome P450 enzymes and other metabolic pathways, leading to increased production of ROS and other free radicals. Pathogenic infections are another trigger of oxidative stress and inflammation for the purpose of immune signaling and eradicating the invaders—a process that, while essential for controlling infection, can lead to tissue damage and disease due to heightened oxidative and inflammatory activity.18 Furthermore, antibiotics to eliminate these pathogens, as well as other medicines that negatively impact the gut microbiome, have been found to increase gut epithelium oxidation due to altered microbial composition and metabolic signaling to the host.23,24 These effects are often amplified by the underlying dysbiosis concurrently induced by the pathogen. The interplay between the myriad of internal and external factors can precipitate a cascade of oxidative and inflammatory responses, underscoring the complexity of maintaining redox equilibrium in the face of diverse challenges.
Antioxidant Factors
Endogenous Antioxidants: The human body employs a multi-tiered defense system against oxidative stress, primarily through endogenous enzymatic and non-enzymatic antioxidants. Genes encode for enzymes such as catalase (CAT), superoxide dismutases (SOD1/2/3), glutathione peroxidase (GPx), and glutathione reductase that act as the first line of defense against ROS.15 These enzymes catalyze reactions that neutralize ROS, thereby preventing cellular damage. For instance, SODs convert superoxide anions into less reactive species like hydrogen peroxide and molecular oxygen.12 Complementing these enzymatic actions are non-enzymatic antioxidants like glutathione, cysteine, and CoQ10, with glutathione being particularly potent in scavenging a variety of reactive compounds.25 Furthermore, the Nrf2-Keap1 system is an evolutionarily conserved intracellular defense mechanism which plays a pivotal role in regulating antioxidant defenses. When activated, the Nrf2 transcription factor translocates to the nucleus and binds to AREs in the DNA, thereby upregulating the expression of various antioxidant genes and proteins, further enhancing the body's ability to counteract oxidative stress.26
Exogenous Antioxidants: Exogenous antioxidants play a vital role in fortifying the body's defenses against oxidative stress, complementing endogenous mechanisms. Dietary sources rich in vitamins C and E, carotenoids, zinc, and selenium contribute to this antioxidative shield by neutralizing free radicals and preventing lipid peroxidation, in addition to supporting antioxidant function of the gut microbiome.27 Notably, polyphenols, a class of plant-based compounds found in foods like fruits, vegetables, spices, and tea, have garnered attention for their potent antioxidative properties.28 The various types of polyphenols (flavonoids, phenolic acids, stilbenes, and lignans) exert their effects through multiple mechanisms—direct scavenging of ROS, modulation of enzymatic activity, interaction with cellular signaling pathways, and modulation of gut microbiome's function and composition.27,29 Their inclusion in the diet can have broad health implications, ranging from delaying aging to mitigating neurodegenerative and cardiometabolic diseases.28 Furthermore, dietary fibers that act as prebiotics and induce beneficial metabolic processes and metabolites from the intestinal microbes serve potent antioxidant functionalities.30 Exogenous antioxidants serve as a crucial adjunct to the body's innate defenses, offering a dietary means of modulating oxidative stress and influencing health outcomes. Their multifaceted actions extend beyond direct antioxidative effects, impacting cellular pathways and gut microbiota, which underscores the interconnectedness of diet, microbial metabolism, and redox balance (Figure 4).
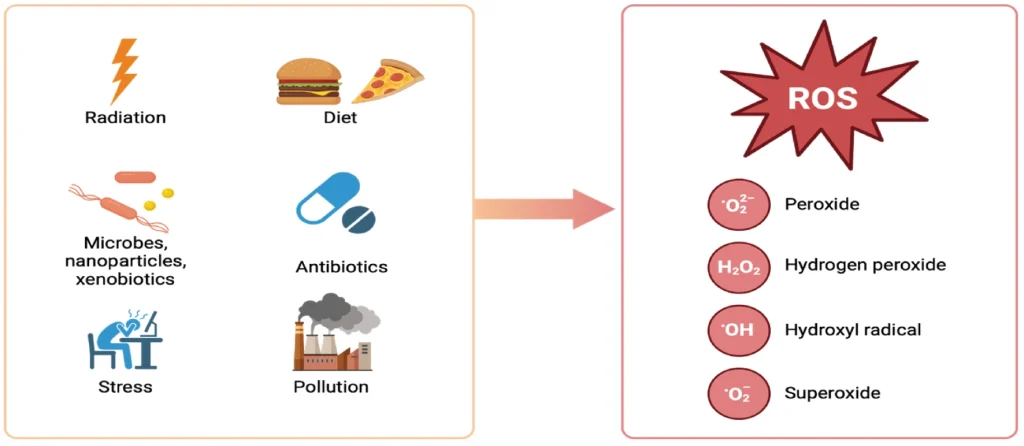
The intricate network of endogenous antioxidants is increasingly compromised by the pressures of modern lifestyles and environmental factors. Enzymatic defenders form a formidable internal defense against the onslaught of oxidation, but this natural protective mechanism can be disrupted by the increasingly common factors of poor dietary choices, pollution, stress, and overuse of antibiotics, leading to a diminished capacity to fend off oxidative damage.27,28 This weakening of the antioxidant defense system is further exacerbated by a reduction in dietary intake of crucial exogenous antioxidants found in fruits, vegetables, and whole foods which support and enhance our body's antioxidative capacity. Moreover, the gut microbiome, an essential player in maintaining redox balance, is adversely affected by these same factors, diminishing its ability to contribute to our antioxidant defenses.29 This decline in both endogenous and exogenous antioxidant capabilities accelerates cellular aging and vulnerability to diseases and underscores the urgent need to address the dietary and environmental factors systematically undermining our body's natural resilience to oxidative stress.22
Redox and the Gut Microbiome
The Gut Microbiome's Broad Role in Health and Disease
The gut microbiota, a complex and dynamic community of microorganisms residing in the GI tract, integrally influences various aspects of host physiology, from nutritional status and behavior to stress response and immunity. This microbial community, which is host specific and evolves over an individual's lifetime, plays a vital role in synthesizing vitamins, digesting dietary components, and protecting against pathogens, among multitude other functions.31 Its contributions to health are profound, impacting not just the local intestinal environment but also systemic physiological processes, and the prevention of disease throughout the body.6-8 When these important connections break down, disruptions of the gut microbiota are linked to a wide array of conditions.14,22
Dysbiosis, or imbalance in the composition and/or function of this microbial community, has been strongly associated with GI diseases6 as well as systemic conditions via its influence on other organs through the numerous gut-organ axes—emphasizing the gut's interconnectedness with broader health processes.32 Such imbalances are implicated in neurological health issues, including Alzheimer's and Parkinson's diseases and autism spectrum disorders, mediated through the gut-brain axis.5 The microbiome's influence on redox processes strongly interplays with its extensive impact on human health and disease.
Bi-Directional Influence of Redox and the Gut Microbiome
The redox environment of the gut is regulated by a dynamic equilibrium between pro-oxidant and antioxidant mechanisms, shaped by the intricate interactions among host cells, the gut microbiota, and dietary factors.33 This equilibrium is essential for maintaining gut homeostasis, impacting cell signaling, energy metabolism, and the integrity of the gut barrier. The redox state significantly influences the gut microbiota's composition and activity, affecting microbial metabolism and their interactions with the host.34 Furthermore, redox processes are a key facilitator of the microbiome's influence on intestinal physiology, shown to mediate symbiosis between the microbiota of the gut and the surrounding intestinal cells.34 An increase in redox potential, indicating elevated oxidation, can lead to dysbiosis; likewise, a shift toward dysbiosis reflects imbalances in redox conditions while simultaneously exacerbating the problem—reflecting the self-reinforcing feedback loop between the microbiota and redox state. Competition among bacteria for electron acceptors serves as a critical ecological determinant within gut communities, as the availability and type of electron acceptors directly affect bacterial respiration types and, consequently, which microbial species dominate.8 This process ultimately influences the gut microbiome's composition and function, underscoring the complex bi-directional relationship between redox processes, microbial ecology, and host health.
Redox Influencing Gut Microbiome's Composition
The redox state in the intestinal environment is shaped by an interplay of host, environmental, and microbial factors, which profoundly impact the structure and function of the gut microbiome. Redox potential is intricately linked to microbial activities and overall intestinal health, serving as a broad indicator of the myriad biochemical processes unfolding within the gut.
Microbial Growth and Composition: The redox environment within the gut, characterized by a gradient of oxygen levels from the small intestine to anoxia in the large intestine, plays a pivotal role in determining microbial growth and community structure. This environment is also influenced by the presence of redox-active molecules like nitric oxide synthase and hydrogen sulfide, affecting the redox potential across different intestinal regions.33 Higher oxygen areas harbor more aerotolerant microbes, aligning with the aerobic or anaerobic metabolic capacities of these organisms. The prevalence of aerotolerant pathogens is often linked to dysbiosis and oxidative stress, with metagenomic indices developed to study these associations.35 Redox dynamics are shaped by dietary inputs, host physiological status, and inflammatory conditions, impacting the microbiota's composition and functionality. Host-derived electron acceptors like oxygen and nitrate, produced during normal physiological processes and increased during disease or inflammation, play a critical role in modulating the gut's microbial landscape.36 Conditions favoring either aerobic or anaerobic bacteria lead to shifts in microbial species dominance, with facultative anaerobes thriving in varied oxygen conditions and obligate anaerobes preferring reduced redox states. Such shifts toward dysbiosis, marked by an abundance of facultatively anaerobic bacteria, indicate alterations in host redox conditions and signal broader physiological changes.8
Microbial Metabolism: The concept of dysbiosis traditionally emphasizes shifts in the composition of bacterial species; however, recent perspectives highlight the value of characterizing dysbiosis via changes in microbial metabolism.8,37 Changes in the redox environment can trigger the expression of oxidative stress response genes, thereby adjusting microbial metabolism to suit the existing conditions. This redox-driven modulation of metabolic pathways affects the production of essential nutrients such as vitamins and amino acids, thereby influencing both microbial and host health. For example, the synthesis of certain B vitamins by gut bacteria, crucial for the host's nutritional well-being and metabolic health, is regulated by the redox state, underscoring the intricate link between microbial metabolism, redox balance, and overall physiological health.
Bacterial Communication and Signaling: Redox conditions critically influence bacterial communication and signaling, particularly through quorum sensing, a mechanism that coordinates gene expression based on population density.38 Alterations in the redox environment can modulate quorum sensing signals, affecting microbial behavior, virulence, and community dynamics. This intricate communication system is foundational to biofilm formation, where oxidative stress can trigger some bacterial species to establish biofilms as protective communities against harsh conditions, including exposure to ROS.
Gut Microbiome Influencing Redox Balance
The gut microbiome is intricately involved in the regulation of redox balance within the GI tract. Gut bacteria can negatively influence redox through interactions with host cells through pattern recognition receptors, leading to an increase in ROS via nicotinamide adenine dinucleotide phosphate (reduced form) oxidases. This overproduction of ROS can alter cellular signaling, induce inflammatory cytokines, and increase epithelial permeability, contributing to chronic inflammation.9 Additionally, some gut bacteria can directly produce ROS, exacerbating inflammation and epithelial damage. Contact between the intestinal epithelium and bacteria such as lactobacilli activates ROS-mediated Keap1/Nrf2/ARE signaling within epithelial cells, triggering important cytoprotective antioxidant mechanisms.34
Beneficial bacteria within a balanced microbiome actively contribute to redox homeostasis. They produce antioxidative enzymes like SOD and CAT, neutralizing ROS and modulating host cell signaling pathways—particularly through the activation of Nrf2, a regulator of the antioxidant response.19 This activation upregulates protective genes encoding detoxifying enzymes and antioxidant proteins, thereby fortifying cellular defenses against oxidative damage. Furthermore, these beneficial bacteria engage in cross talk with immune cells, fine-tuning immune responses and maintaining a balance between pro-inflammatory and anti-inflammatory cytokines.39 This balance is crucial for preventing an overactive immune response that can lead to chronic inflammation and associated oxidative stress. By promoting the proliferation of regulatory T cells and the production of anti-inflammatory cytokines, these microbes help establish a controlled immune environment less likely to trigger excessive ROS production.9 Together, these interactions within the gut microbiome underscore its essential role in maintaining redox equilibrium, not just within the gut but throughout the body, emphasizing its significance in addressing chronic illnesses linked to gut dysbiosis.
Antioxidant Microbial Metabolites
The microbiome plays a pivotal role in systemic redox balance by generating a variety of antioxidant metabolites, such as fatty acids, secondary bile acids, sphingolipids, polyamines, tryptophan, and indole derivatives. These metabolites exert their effects not only within the gut but also across various organs due to their capacity to enter systemic circulation.
Antioxidant Response Elements
AREs are DNA sequences that regulate the expression of genes encoding for antioxidant enzymes within our human cells, such as SOD, CAT, and GPx. The activation of AREs is primarily controlled by the Nrf2/Keap1 signaling pathway, which plays a central role in cellular defense against oxidative stress. Recent research indicates that microbial metabolites, particularly those produced by gut bacteria, can directly influence ARE activation. Additionally, it has been shown that the Nrf2/ARE pathway is crucial not only in redox homeostasis but also in the management of inflammatory diseases such as inflammatory bowel disease (IBD).40
The Nrf2/ARE pathway, by inducing antioxidant and anti-inflammatory responses, contributes significantly to the alleviation of oxidative stress and inflammation in the gut, thus playing a protective role in conditions like IBD. Furthermore, natural products, such as polyphenols, terpenoids, and alkaloids, are likely converted by gut bacteria into bioactive metabolites that can activate the Nrf2/ARE pathway, enhancing the host's antioxidant capacity and contributing to the management of oxidative stress-related diseases.41
Short-Chain Fatty Acids
In particular, the effects of short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate are central to the regulation of redox homeostasis within the gut. In fact, a growing body of evidence illustrates that direct supplementation of fiber, the raw material that bacteria biotransform into metabolites, during microbiome-impacting treatment such as antibiotics can help to alleviate associated redox dysregulation, helping to protect commensal microbes during treatment.30 SCFAs contribute significantly to the structural maintenance of the intestinal barrier by enhancing the function of tight junction proteins through these AREs, which are crucial for barrier integrity and the prevention of pathogen translocation. These compounds modulate Nrf2-Keap1, signaling pathways that are central to redox and inflammatory regulation throughout the body.42 They enhance tight junction protein functionality and modulate redox balance via oxygen-consuming reactions in the intestinal epithelium, stabilizing hypoxia-inducible factors (HIFs) essential for barrier integrity.23 Importantly, microbiome-produced butyrate induces O2− consuming reactions in the intestinal epithelium, modulating the redox balance and stabilizing HIF, a transcription factor involved in barrier protection.43
Moreover, SCFAs play a protective role in mitochondrial health, shielding these energy-generating organelles from oxidative stress and the resultant damage. They also contribute to the regulation of key signaling pathways and transcription factors involved in mitochondrial biogenesis and function, such as peroxisome proliferator-activated receptor gamma coactivator 1-alpha, sirtuin 1, and adenosine monophosphate-activated protein kinase, enhancing the energy-generating capacity of mitochondria and protect against ROS-induced mitochondrial damage.44 By improving mitochondrial function and metabolism, SCFAs help maintain an efficient energy supply to the cells, which is essential for various cellular activities and overall vitality. This mitochondrial protection is crucial in preventing the initiation of a harmful cycle where mitochondrial damage leads to increased ROS production, which in turn causes further mitochondrial impairment. The antioxidative properties of SCFAs are thus a key element in the prevention of oxidative stress-related cellular damage, contributing to the maintenance of redox balance both within the gut environment and systemically. The intricate relationship between these microbial metabolites and the host's redox biology underscores the profound impact of the gut microbiome on overall health, emphasizing the potential of targeted dietary and probiotic interventions to harness these benefits for improved health outcomes.
Other Bacterial Metabolites
Bacterial-derived sphingolipids such as ceramides actively regulate cellular redox homeostasis and inflammation.45 While excess primary (liver-produced) bile acids can cause DNA damage, the secondary bile acids such as ursodeoxycholic acid and tauroursodeoxycholic acid, which are produced by microbial metabolism of the primary bile acids, have antioxidant properties.46,47 These acids are increasingly studied for their potential in treating oxidative stress-related conditions, including Barrett's esophagus, neurodegenerative diseases, and liver disease.48 Dietary and bacteria-derived amino acids like tryptophan, methionine, histidine, lysine, cysteine, arginine, and tyrosine display notable antioxidant properties.49 Microbial metabolism of amino acids can also create derivatives with enhanced antioxidant and signaling potential. Tryptophan derivatives, such as 5-hydroxytryptamine, melatonin, xanthurenic acid, 5-methoxyindoleacetic acid, and indole derivatives, effectively scavenge oxidative molecules, regulating ROS pathways and increasing Nrf2 expression.50 Indole derivatives, including indole-3-propionic acid and indole acetic acid, are extensively researched for their antioxidative and anti-inflammatory activities. They play diverse roles, from improving tight junction expression to modulating immune function and exerting regulatory effects on organ-specific systems through gut-organ axes.51,52 These derivatives are particularly promising for oxidative stress-related diseases, including neurological disorders like Alzheimer's disease, due to their increased ability to cross the blood-brain barrier.53
The gut microbiota further produces a diverse range of antioxidant molecules by metabolizing dietary compounds, especially polyphenols, ellagitannins, and other plant-derived molecules.54 Indole derivatives, metabolites known for their antioxidative and anti-inflammatory effects, are shown to improve gut barrier functions, modulate immunity, and influence organ systems via gut-organ axes.51,52 Their ability to cross the blood-brain barrier underscores their potential in treating oxidative stress-related neurological disorders.51,53 The comprehensive impacts of these microbial metabolites suggest an immense therapeutic potential of a material that could harness the entirety of the gut microbiome's metabolic output.
Modulating the Microbiome for Redox Benefit
While a “healthy” gut microbiome does not conform to a single composition, a health-associated microbial ecosystem in the human gut typically exhibits several defining characteristics: high alpha diversity, stability over time, resilience to disturbances, resistance to pathogen colonization, and robust metabolic output that supports the health of the host cells as well as the microbial ecosystem.55 Such a eubiotic state contributes significantly to host health by resisting pathogens, supplying essential nutrients, and maintaining metabolic and immunologic homeostasis, in addition to playing a crucial role in maintaining redox balance throughout the body.34,55 Current strategies to foster microbiome health include dietary interventions, increasing fiber intake, and the use of prebiotics and probiotics.
Diet is the primary source of exogenous antioxidant components but also fundamentally shapes the structure, composition, and functionality of the gut microbiome, serving as the most effective tool for influencing microbiome health.56 Dietary components, especially plant-derived fibers, nutrients, vitamins, and micronutrients, serve as substrates for bacterial growth and metabolism, in turn modulating the redox state of the gut. The diversity, quality, and plant content of foods consumed directly affect the diversity and metabolic output of gut bacteria; a diverse and balanced diet supports a robust microbiome capable of effectively managing oxidative stress and maintaining redox homeostasis.29 Conversely, diets high in processed foods and low in fiber lead to a disrupted balance, reducing microbial diversity and impairing the redox state, often resulting in negative health outcomes such as further increased oxidative stress and inflammation.
This regulation is crucial, as a well-regulated redox environment protects the intestinal lining, supports immune function, and prevents chronic inflammation. This self-reinforcing feedback loop involving diet, microbiome, and intestinal health via the redox state is reflected in recent studies showing that fiber prebiotics significantly reduced the impact of antibiotic treatment on microbiome's composition and function via modulation of the gut redox potential.30 Therefore, dietary choices are instrumental in managing gut microbiome health and are used as a key tool for influencing the gut microbiome metabolism, redox state, and intestinal health.
Probiotics, specifically defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host,”57 are pivotal tools for positively modulating the microbiome's composition and function, thereby influencing redox homeostasis both locally and systemically. This benefit is largely due to the antioxidant activities facilitated by a eubiotic microbiome and is often closely correlated with the metabolic output of the microbiota.50 Moreover, probiotics exhibit direct, strain-specific antioxidant properties through various mechanisms, which have been extensively reviewed in recent literature.19,20,50, 58, 59 Internal mechanisms include the production of their own antioxidant enzymes like SOD and CAT and synthesis of antioxidant metabolites such as folate and glutathione. Additionally, probiotics act on the surrounding environment and the host, enhancing host antioxidant activities, chelating metal ions, reducing activity of ROS-producing enzymes, and modulating key signaling pathways involved in oxidative stress such as Nrf2-Keap1-ARE, nuclear factor kappa-light-chain-enhancer of activated B cells, mitogen-activated protein kinase, and protein kinase C.20,59,60 In vitro studies have demonstrated that multiple components of bacteria contribute antioxidant functionality, including the culture supernatant, intact cells, and cell-free extracts.20 This diverse range of functionalities underscores the direct antioxidant capacity of probiotics as well as their integral role in maintaining oxidative balance of their surroundings and host. It must be noted that the specific probiotic effects described in the literature are based upon specific probiotic species, strains, combinations, and dosages.
Fecal Transplant as a Comprehensive Microbiome Modulator
Fecal microbiota transplant (FMT) is increasingly shown to be a powerful strategy for the restoration and maintenance of a healthy gut microbiota, leveraging the inherent richness of healthy fecal material in beneficial microbes as well as microbiota- and redox-modulating compounds. FMT involves transferring fecal matter from a healthy donor to a recipient, with the goal of modulating the gut ecosystem toward homeostasis. This technique has demonstrated exceptional effectiveness in treating conditions like Clostridium difficile infection and is being clinically investigated for its broader potential in an extensive list of conditions, including IBD, liver disease, neurodegeneration, graft-versus-host disease, metabolic syndrome, and more.22, 61, 62 Furthermore, pre-clinical models increasingly demonstrate the impact of FMT on modulating redox-related processes and associated conditions.25,63
FMT Challenges
Standardization
One of the foremost challenges with FMT is standardization. While pooling stools from different donors over time has been proposed to normalize variability, this approach introduces additional infectious risks and limits traceability. Furthermore, the efficacy of FMT varies significantly between donors and procedures.64
Safety
Fecal transplantation entered the US medical paradigm with the initial hypothesis that live bacterial engraftment was essential for its effectiveness, particularly against pathogenic C. difficile infections. This modality, however, comes with inherent risks of transmitting fecal-oral infectious agents—bacterial, viral, and fungal—from donor to recipient. Initial screening protocols for fecal donors, modeled after those for blood donation, proved inadequate when a strain of pathogenic Escherichia coli—benign in a healthy donor but lethal in immunocompromised recipients—led to cases of sepsis and death.65 The COVID-19 pandemic added another layer to this issue, demonstrating that it is impractical to pre-emptively test for every conceivable infectious agent in untreated stool.
Mechanism
While the efficacy of FMT is well documented, the exact mechanisms underlying its ability to shift the microbial ecosystem are not fully understood. The predominant theory suggests that the successful transplantation and engraftment of the donor's microbiota into the recipient's gut is essential.64,66 However, this view is challenged by the effectiveness of fecal filtrate preparations lacking live bacteria that still manage to alter the recipient's microbiota and treat recurrent C. difficile infection.67 This indicates that metabolites, bacterial components, bacteriophages, bioactive lipids, and/or other fecal components may also play significant roles. Therefore, it is imperative to investigate alternative mechanisms that contribute to FMT's efficacy, moving beyond the activity of live bacteria and enabling the development of synthetic and targeted therapeutic applications.
Metabolites as FMT Active Agents
The broad range of metabolites in fecal material, encompassing significant antioxidant, redox-modulating, and anti-inflammatory functions, increasingly points to the vital role of metabolites and other non-live, microbiome-derived molecules in FMT efficacy. Importantly, many of these bacterial metabolites are effective at relatively low concentrations.68 While defining an “optimal” microbiome composition is challenging due to individual variability and heterogeneity, focusing on metabolite profiles provides a more consistent and stable metric for understanding microbiome impacts.69 This approach shifts the focus from merely identifying microbial presence to understanding microbial functionality.
Metabolite Postbiotics
Microbial metabolites fall within the group classified as “postbiotics,” an emerging, evolving term that encompasses both inanimate microorganisms and their components that confer health benefits,70 as well as the myriad of metabolites generated through fermentation and bacteria-host interactions.71 Postbiotics have been shown to modulate a wide array of cellular and molecular mechanisms, displaying anti-inflammatory, immunomodulatory, anti-obesogenic, and anti-dysbiotic properties.72-75 Importantly, they impact not only the gut, where they serve as substrates for microbes and human cells, but also influence distant systems via the many gut-organ axes. While encouraging, most research has been limited to postbiotics from single bacterial cultures, representing only a fraction of the metabolite spectrum and outside the context of consortial metabolic interactions within the gut ecosystem. With the microbiome producing an estimated 1000-10,000 molecules affecting human physiology76 and only 20%-40% of the gut's 500-1000 bacterial species being culturable,75 reliance on isolated bacterial strains overlooks the wider therapeutic potential available from complex microbial mixtures. The therapeutic potential of microbial metabolites is a growing topic of investigation,77 and the human gut microbiome presents a largely untapped resource for microbial metabolites and other postbiotics, offering an alternative to the limitations of using isolated bacterial strains.
Conclusion
The gut microbiome has emerged as a pivotal player in redox balance, with its dysbiosis contributing to systemic oxidative stress and inflammation, thereby influencing the progression of a wide array of diseases. Additionally, the role of oxidative stress, secondary to numerous physiologic imbalances and environmental inputs, affects the structure and function of the gut microbiome.78 This can lead to a “vicious cycle” of imbalance, dysbiosis, and disease. The body's defense against this oxidative onslaught is multilayered, involving a host of endogenous antioxidants and enzymes, as well as exogenous dietary factors that provide additional support. The gut microbiome's role is highlighted in its ability to produce antioxidative enzymes and metabolites, which contribute to maintaining redox balance. These microbial metabolic products influence systemic physiological processes by supporting the structural integrity of the intestinal barrier, decreasing low-grade inflammation, and playing a protective role in mitochondrial health. It is essential that we bring awareness to the role of the gut microbiome in redox balance and harness the antioxidant capabilities of myriad microbial metabolites. This area of focus represents a unique approach to mitigating oxidative stress and promoting redox equilibrium, with implications for enhancing human health and managing disease.
Conflicts of Interest
AM is supported by Thaena Inc., and EM is supported by Thaena Inc. and Seed.
Funding
HZ and BT are supported by NIDDK R25DK130848-01A1 and NCCIH R90AT008924-09.
Authors' Contributions
AM: conceptualization, methodology, writing; AT: literature searches, formatting, and figure preparation; BT: conceptualization, methodology; PH: conceptualization, methodology; EM: methodology, writing original draft; HZ: methodology, writing review and editing.
References
- Dalton TP, Shertzer HG, Puga A. Regulation of gene expression by reactive oxygen. Annu Rev Pharmacol Toxicol. 1999;39(1):67-101. https://doi.org/10.1146/annurev.pharmtox.39.1.67.
- Kim S. Reduction-oxidation reaction (redox reaction). BioRender; 2023. https://www.biorender.com/template/reduction-oxidation-reaction-redox-reaction Accessed on Sept. 20, 2024.
- Pizzino G, Irrera N, Cucinotta M, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. 2017;2017(1):8416763. https://doi.org/10.1155/2017/8416763.
- Catherine C. Sources of reactive oxygen species (ROS) with cell background. BioRender; 2022. https://www.biorender.com/template/sources-of-reactive-oxygen-species-ros-with-cell-background Accessed on Sept. 20, 2024.
- Cryan JF, O'Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179-94. https://doi.org/10.1016/S1474-4422(19)30356-4.
- Nishida A, Inoue R, Inatomi O, et al. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11(1):1-10. https://doi.org/10.1007/s12328-017-0813-5.
- Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55-71. https://doi.org/10.1038/s41579-020-0433-9.
- Winter SE, Bäumler AJ. Gut dysbiosis: ecological causes and causative effects on human disease. Proc Natl Acad Sci. 2023;120(50):e2316579120. https://doi.org/10.1073/pnas.2316579120.
- Kunst C, Schmid S, Michalski M, et al. The influence of gut microbiota on oxidative stress and the immune system. Biomedicines. 2023;11(5):1388. https://doi.org/10.3390/biomedicines11051388.
- Ziada AS, Smith MSR, Côté HCF. Updating the free radical theory of aging. Front Cell Dev Biol. 2020;8:575645. https://doi.org/10.3389/fcell.2020.575645.
- Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94(2):329-54. https://doi.org/10.1152/physrev.00040.2012.
- Campbell EL, Colgan SP. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2019;16(2):106-20. https://doi.org/10.1038/s41575-018-0079-5.
- Seyedsadjadi N, Grant R. The potential benefit of monitoring oxidative stress and inflammation in the prevention of non-communicable diseases (NCDs). Antioxidants. 2020;10(1):15. https://doi.org/10.3390/antiox10010015.
- Li Z, Xu D, Li X, et al. Redox imbalance in chronic inflammatory diseases. Biomed Res Int. 2022;2022:1-3. https://doi.org/10.1155/2022/9813486.
- Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. 2021;20(9):689-709. https://doi.org/10.1038/s41573-021-00233-1.
- Korac B, Kalezic A, Pekovic-Vaughan V, et al. Redox changes in obesity, metabolic syndrome, and diabetes. Redox Biol. 2021;42:101887. https://doi.org/10.1016/j.redox.2021.101887.
- Jiang W. Sources of oxidants and antioxidants. BioRender; 2023. https://www.biorender.com/template/sources-of-oxidants-and-antioxidants Accessed on Sept. 20, 2024.
- Yang X, Liu X, Nie Y, et al. Oxidative stress and ROS-mediated cellular events in RSV infection: potential protective roles of antioxidants. Virol J. 2023;20(1):224. https://doi.org/10.1186/s12985-023-02194-w.
- Feng T, Wang J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: a systematic review. Gut Microbes. 2020;12(1):1801944. https://doi.org/10.1080/19490976.2020.1801944.
- Wang Y, Wu Y, Wang Y, et al. Antioxidant properties of probiotic bacteria. Nutrients. 2017;9(5):521. https://doi.org/10.3390/nu9050521.
- Nolfi-Donegan D, Braganza A, Shiva S. Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020;37:101674. https://doi.org/10.1016/j.redox.2020.101674.
- Wang H, Yang F, Zhang S, et al. Genetic and environmental factors in Alzheimer's and Parkinson's diseases and promising therapeutic intervention via fecal microbiota transplantation. NPJ Parkinsons Dis. 2021;7(1):70. https://doi.org/10.1038/s41531-021-00213-7.
- Kelly CJ, Zheng L, Campbell EL, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17(5):662-71. https://doi.org/10.1016/j.chom.2015.03.005.
- Reese AT, Cho EH, Klitzman B, et al. Antibiotic-induced changes in the microbiota disrupt redox dynamics in the gut. eLife. 2018;7:e35987. https://doi.org/10.7554/eLife.35987.
- Singh V, Ahlawat S, Mohan H, et al. Balancing reactive oxygen species generation by rebooting gut microbiota. J Appl Microbiol. 2022;132(6):4112-29. https://doi.org/10.1111/jam.15504.
- Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865(5):721-33. https://doi.org/10.1016/j.bbamcr.2018.02.010.
- Naliyadhara N, Kumar A, Gangwar SK, et al. Interplay of dietary antioxidants and gut microbiome in human health: what has been learnt thus far? J Funct Foods. 2023;100:105365. https://doi.org/10.1016/j.jff.2022.105365.
- García-Aguilar A, Palomino O, Benito M, Guillén C. Dietary polyphenols in metabolic and neurodegenerative diseases: molecular targets in autophagy and biological effects. Antioxidants. 2021;10(2):142. https://doi.org/10.3390/antiox10020142.
- Riaz Rajoka MS, Thirumdas R, Mehwish HM, et al. Role of food antioxidants in modulating gut microbial communities: novel understandings in intestinal oxidative stress damage and their impact on host health. Antioxidants. 2021;10(10):1563. https://doi.org/10.3390/antiox10101563.
- Penumutchu S, Korry BJ, Hewlett K, Belenky P. Fiber supplementation protects from antibiotic-induced gut microbiome dysbiosis by modulating gut redox potential. Nat Commun. 2023;14(1):5161. https://doi.org/10.1038/s41467-023-40553-x.
- Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859-904. https://doi.org/10.1152/physrev.00045.2009.
- Ahlawat S, Asha, Sharma KK. Gut-organ axis: a microbial outreach and networking. Lett Appl Microbiol. 2021;72(6):636-68. https://doi.org/10.1111/lam.13333.
- Circu ML, Aw TY. Redox biology of the intestine. Free Radic Res. 2011;45(11-12):1245-66. https://doi.org/10.3109/10715762.2011.611509.
- Neish AS, Jones RM. Redox signaling mediates symbiosis between the gut microbiota and the intestine. Gut Microbes. 2014;5(2):250-3. https://doi.org/10.4161/gmic.27917.
- Million M, Raoult D. Linking gut redox to human microbiome. Hum Microbiome J. 2018;10:27-32. https://doi.org/10.1016/j.humic.2018.07.002.
- Winter SE, Thiennimitr P, Winter MG, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467(7314):426-9. https://doi.org/10.1038/nature09415.
- Heinken A, Hertel J, Thiele I. Metabolic modelling reveals broad changes in gut microbial metabolism in inflammatory bowel disease patients with dysbiosis. NPJ Syst Biol Appl. 2021;7(1):19. https://doi.org/10.1038/s41540-021-00178-6.
- Bakovic A, Risner K, Bhalla N, et al. Brilacidin demonstrates inhibition of SARS-CoV-2 in cell culture. Viruses. 2021;13(2):271. https://doi.org/10.3390/v13020271.
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121-41. https://doi.org/10.1016/j.cell.2014.03.011.
- Khor TO, Huang MT, Kwon KH, et al. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66(24):11580-4. https://doi.org/10.1158/0008-5472.CAN-06-3562.
- Li B, Wang Y, Jiang X, et al. Natural products targeting Nrf2/ARE signaling pathway in the treatment of inflammatory bowel disease. Biomed Pharmacother. 2023;164:114950. https://doi.org/10.1016/j.biopha.2023.114950.
- González-Bosch C, Zunszain PA, Mann GE. Control of redox homeostasis by short-chain fatty acids: implications for the prevention and treatment of breast cancer. Pathogens. 2023;12(3):486. https://doi.org/10.3390/pathogens12030486.
- Kelly JR, Clarke G, Harkin A, et al. Seeking the psilocybiome: psychedelics meet the microbiota-gut-brain axis. Int J Clin Health Psychol. 2023;23(2):100349. https://doi.org/10.1016/j.ijchp.2022.100349.
- Clark A, Mach N. The crosstalk between the gut microbiota and mitochondria during exercise. Front Physiol. 2017;8:319. https://doi.org/10.3389/fphys.2017.00319.
- Won JS, Singh I. Sphingolipid signaling and redox regulation. Free Radic Biol Med. 2006;40(11):1875-88. https://doi.org/10.1016/j.freeradbiomed.2006.01.035.
- Lapenna D, Ciofani G, Festi D, et al. Antioxidant properties of ursodeoxycholic acid. Biochem Pharmacol. 2002;64(11):1661-7. https://doi.org/10.1016/S0006-2952(02)01391-6.
- Daruich A, Picard E, Boatright JH, Behar-Cohen F. Review: the bile acids urso- and tauroursodeoxycholic acid as neuroprotective therapies in retinal disease. Mol Vis. 2019;25:610-24.
- Peng S, Huo X, Rezaei D, et al. In Barrett's esophagus patients and Barrett's cell lines, ursodeoxycholic acid increases antioxidant expression and prevents DNA damage by bile acids. Am J Physiol Gastrointest Liver Physiol. 2014;307(2):G129-39. https://doi.org/10.1152/ajpgi.00085.2014.
- Xu K, Liu H, Bai M, et al. Redox properties of tryptophan metabolism and the concept of tryptophan use in pregnancy. Int J Mol Sci. 2017;18(7):1595. https://doi.org/10.3390/ijms18071595.
- Zhao J, Zhao F, Yuan J, et al. Gut microbiota metabolites, redox status, and the related regulatory effects of probiotics. Heliyon. 2023;9(11):e21431. https://doi.org/10.1016/j.heliyon.2023.e21431.
- Jiang H, Chen C, Gao J. Extensive summary of the important roles of indole propionic acid, a gut microbial metabolite in host health and disease. Nutrients. 2022;15(1):151. https://doi.org/10.3390/nu15010151.
- Oliveira DL, Pugine SMP, Ferreira MSL, et al. Influence of indole acetic acid on antioxidant levels and enzyme activities of glucose metabolism in rat liver. Cell Biochem Funct. 2007;25(2):195-201. https://doi.org/10.1002/cbf.1307.
- Bendheim PE, Poeggeler B, Neria E, et al. Development of indole-3-propionic acid (OXIGON™) for Alzheimer's disease. J Mol Neurosci. 2002;19(1-2):213-7. https://doi.org/10.1007/s12031-002-0036-0.
- Rani A, Saini K, Bast F, et al. Microorganisms: a potential source of bioactive molecules for antioxidant applications. Molecules. 2021;26(4):1142. https://doi.org/10.3390/molecules26041142.
- Wilmanski T, Diener C, Rappaport N, et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab. 2021;3(2):274-86. https://doi.org/10.1038/s42255-021-00348-0.
- Zhang P. Influence of foods and nutrition on the gut microbiome and implications for intestinal health. Int J Mol Sci. 2022;23(17):9588. https://doi.org/10.3390/ijms23179588.
- Hill C, Guarner F, Reid G, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506-14. https://doi.org/10.1038/nrgastro.2014.66.
- Mishra V, Shah C, Mokashe N, et al. Probiotics as potential antioxidants: a systematic review. J Agric Food Chem. 2015;63(14):3615-26. https://doi.org/10.1021/jf506326t.
- Kong Y, Olejar KJ, On SLW, Chelikani V. The potential of Lactobacillus spp. for modulating oxidative stress in the gastrointestinal tract. Antioxidants. 2020;9(7):610. https://doi.org/10.3390/antiox9070610.
- Oniszczuk A, Oniszczuk T, Gancarz M, Szymańska J. Role of gut microbiota, probiotics and prebiotics in the cardiovascular diseases. Molecules. 2021;26(4):1172. https://doi.org/10.3390/molecules26041172.
- Hassouneh R, Bajaj JS. Gut microbiota modulation and fecal transplantation: an overview on innovative strategies for hepatic encephalopathy treatment. J Clin Med. 2021;10(2):330. https://doi.org/10.3390/jcm10020330.
- Feng J, Chen Y, Liu Y, et al. Efficacy and safety of fecal microbiota transplantation in the treatment of ulcerative colitis: a systematic review and meta-analysis. Sci Rep. 2023;13(1):14494. https://doi.org/10.1038/s41598-023-41182-6.
- Zhou J, Hao J, Zhong Z, et al. Fecal microbiota transplantation in mice exerts a protective effect against doxorubicin-induced cardiac toxicity by regulating Nrf2-mediated cardiac mitochondrial fission and fusion. Antioxid Redox Signal. 2024;41(1-3):1-23. https://doi.org/10.1089/ars.2023.0355.
- Wilson BC, Vatanen T, Cutfield WS, O'Sullivan JM. The super-donor phenomenon in fecal microbiota transplantation. Front Cell Infect Microbiol. 2019;9:2. https://doi.org/10.3389/fcimb.2019.00002.
- Burstiner LS, Silver J, Burstiner LJ, et al. Escherichia coli O157: H7 sepsis following fecal microbiota transplant in an IgA-deficient inflammatory bowel disease patient. Gastroenterol Rep. 2022;10:goab041. https://doi.org/10.1093/gastro/goab041.
- Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. 2016;13(9):508-16. https://doi.org/10.1038/nrgastro.2016.98.
- Ott SJ, Waetzig GH, Rehman A, et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology. 2017;152(4):799-811.e7. https://doi.org/10.1053/j.gastro.2016.11.010.
- Sonowal R, Swimm A, Sahoo A, et al. Indoles from commensal bacteria extend healthspan. Proc Natl Acad Sci U S A. 2017;114(36):E7506-E7515. https://doi.org/10.1073/pnas.1706464114.
- Visconti A, Le Roy CI, Rosa F, et al. Interplay between the human gut microbiome and host metabolism. Nat Commun. 2019;10(1):4505. https://doi.org/10.1038/s41467-019-12476-z.
- Salminen S, Collado MC, Endo A, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021;18(9):649-67. https://doi.org/10.1038/s41575-021-00440-6.
- Peluzio MDCG, Martinez JA, Milagro FI. Postbiotics: metabolites and mechanisms involved in microbiota-host interactions. Trends Food Sci Technol. 2021;108:11-26. https://doi.org/10.1016/j.tifs.2020.12.004.
- Jastrząb R, Graczyk D, Siedlecki P. Molecular and cellular mechanisms influenced by postbiotics. Int J Mol Sci. 2021;22(24):13475. https://doi.org/10.3390/ijms222413475.
- Tanaka Y, Hirose Y, Yamamoto Y, et al. Daily intake of heat-killed Lactobacillus plantarum L-137 improves inflammation and lipid metabolism in overweight healthy adults: a randomized-controlled trial. Eur J Nutr. 2020;59(6):2641-9. https://doi.org/10.1007/s00394-019-02112-3.
- Miyazawa K, Kawase M, Kubota A, et al. Heat-killed Lactobacillus gasseri can enhance immunity in the elderly in a double-blind, placebo-controlled clinical study. Benef Microbes. 2015;6(4):441-50. https://doi.org/10.3920/BM2014.0108.
- Walker AW, Duncan SH, Louis P, Flint HJ. Phylogeny, culturing, and metagenomics of the human gut microbiota. Trends Microbiol. 2014;22(5):267-74. https://doi.org/10.1016/j.tim.2014.03.001.
- Piqué N, Berlanga M, Miñana-Galbis D. Health benefits of heat-killed (tyndallized) probiotics: an overview. Int J Mol Sci. 2019;20(10):2534. https://doi.org/10.3390/ijms20102534.
- Descamps HC, Herrmann B, Wiredu D, Thaiss CA. The path toward using microbial metabolites as therapies. EBioMedicine. 2019;44:747-54. https://doi.org/10.1016/j.ebiom.2019.05.063.
- Han M, Lee D, Lee SH, Kim TH. Oxidative stress and antioxidant pathway in allergic rhinitis. Antioxidants (Basel). 2021;10(8):1266. https://doi.org/10.3390/antiox10081266.