Abstract
A 57-year-old White female was diagnosed with high low-density lipoprotein cholesterol (LDL-C). By manipulating her diet, she was able to reduce her LDL-C from its highest level of 262 mg/dL to 138 mg/dL and her total cholesterol from 317 to 229 mg/dL. This approximately 50% reduction was achieved within a 6-month period. By researching the biochemical processes of foods that lower cholesterol, we were able to identify the ideal combinations of food types, consumption frequencies, and amounts that led to the decreased LDL-C. We found the effects of these foods on cholesterol have already been researched and established for years. The subject kept daily diet logs for an entire year, through which we could approximately quantify the consumption of saturated fat and cholesterol, foods with phytosterols, and overall calorie intake. This case implies that diet prescription alone may reduce LDL-C for some individuals. The consideration of cholesterol-lowering medications should not overshadow the importance of the well-researched effects that certain foods have on lowering LDL-C and the benefit they have for an individual’s overall health.
Introduction
Cardiovascular disease (CVD) is the leading cause of death according to the Centers for Disease Control (CDC). The main risk marker for CVD is low-density lipoprotein (LDL) levels above 100 mg/dL. At this level LDL molecules are at increased risk of oxidation, which can lead to atherosclerosis. The revised 2018 guideline from the 2018 American Heart Association (AHA) and American College of Cardiologists (ACC), i.e., the Multisociety Guideline on the Management of Blood Cholesterol, recommends that all individuals follow a heart-healthy lifestyle and that adults with LDL-cholesterol (LDL-C) levels greater than 190 mg/dL, with or without atherosclerotic cardiovascular disease (ASCVD) risk, start a statin therapy.1 Statin therapy lowers LDL-C in the blood by inhibiting PCSK9 and increasing the number of LDL receptors; unfortunately, there is uncertainty about its long-term safety, i.e., if used for longer than 3 years.
In the United States, 35 million individuals (20% of whom are women) are using statin drugs to reduce LDL-C levels. According to the American Medical Association, one in five people refuse to use medication and opt for lifestyle and diet changes. Research indicates that dietary changes can reduce cholesterol levels.2 These dietary changes are especially effective when accompanied by physical exercise. Case reports such as this one, that show improved LDL-C through dietary changes, can provide foundational evidence and rationale for larger clinical trials to investigate whether diet and lifestyle changes before medication may be sufficient for lowering cholesterol.
We report a case of a 57-year-old White female who was diagnosed with high cholesterol levels and has a family history of CVD. She was advised to start drug therapy but refused and chose instead to change her diet. When her cholesterol increased after making some dietary changes, she briefly consulted a registered dietitian. Unsatisfied with the dietitian’s recommendations, she further educated herself about diet, and over the next 6 months was able to reduce her LDL-C by approximately 50%. She cut down on foods that contained saturated fat, cholesterol, and excess calories, and she consumed a consistent weekly quantity of phytosterol-rich foods such as nuts and natural peanut butter. This case illustrates that diet alone in some individuals is sufficient to lower elevated cholesterol levels. Further, this case underscores the need for ongoing nutritional counseling to expedite favorable results.
Case History
A 57-year-old White female was diagnosed by her cardiologist with high LDL-C (197 mg/dL, Figure 1) and total cholesterol (317 mg/dL, Figure 1). At age 47 years she was diagnosed with hyperlipidemia by her gynecologist but chose not to pursue any changes; she did not think it was a problem. She had also been counseled by other doctors about her high cholesterol, but she continued to believe high cholesterol was not a problem until her father, a non-smoker and non-alcohol consumer, had a heart attack at the age of 79 years. When her father’s doctor played a video of the inserted stent showing the amount of plaque that was in the artery, she finally addressed her high cholesterol.
Figure 1: Mean blood lipid test results for three distinct diet periods.
The initial test was conducted prior to the diet change. The second test result is after high-cholesterol diet. The third test result is after low-cholesterol diet. HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein.
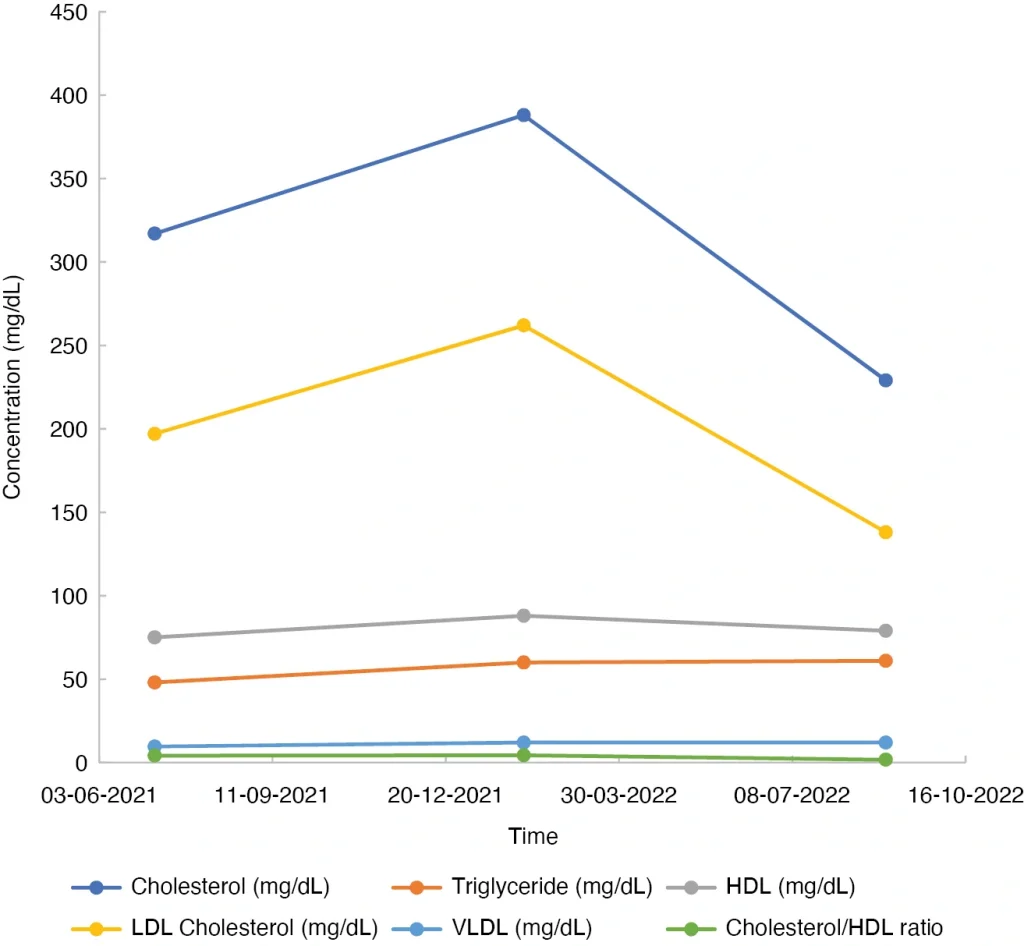
Her cardiologist suggested she start medication to lower her cholesterol. Unwilling to be on medication, she pursued dietary changes instead. The largest portion of her diet comprised dairy and meats, and she enjoyed them daily. Without professional nutritional advice within the first 6 months of modifying her diet, she was misinformed by friends that high-quality, grass-fed meats would not raise her cholesterol. Her high-cholesterol diet (HCD), when analyzed, included an abundance of high-quality, grass-fed animal products and free-range eggs (Table 1). This diet, i.e., her abundant intake of animal products that contain cholesterol and saturated fat, drove her LDL-C up to 262 mg/dL and her total cholesterol to 388 mg/dL (Figure 1), even though she consumed foods containing pectin, beta glucan, and phytosterol that are known to lower cholesterol.2
Table 1: High-cholesterol (LDL-C 197 mg/dL, TC 317 mg/dL) diet sample dates: August 1, 2021 to August 7, 2021a.
| Sunday | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday |
|---|---|---|---|---|---|---|
| 5 C curry vegetables | 2 eggs | 2 eggs | 2 eggs | 4¼ oz sardines | 1 C spinach | 8 oz. V8 juice |
| ½ C GF ground beef | ½ C GF ground beef | ½ avocado | ½ C mushrooms | ¼ C marinara sauce | 2 eggs | ¼ onion |
| ¼ avocado | 1 slice cheddar cheese | ¼ C + 10 raspberries | 1 C spinach | 2 eggs | 1 slice pepper jack cheese | ¼ C mushrooms |
| ½ C raspberries | 1 small gluten-free hot dog | ½ C plain Greek full-fat yogurt | 1 tbsp GF butter | ¼ C mushrooms | 1 C curry vegetables | 3 oz. salmon |
| ½ C blueberries | ¼ C sauerkraut | ¼ onion | 1 slice pepper jack cheese | ¼ C ground beef | ½ C chicken strips | 1 C spinach |
| 1 C almond-coconut milk | ½ C raspberries | 1 C curry vegetables | ¾ C strawberries | ½ C spinach | ¼ onion | 8 oz. V8 juice |
| 1 Americano coffee | 1 slice cheddar cheese | 1 pc. Lindt 95% chocolate | 1 C goat yogurt | 1 tbsp parmesan | ½ C oatmeal | ¼ onion |
| 2 tbsp half & half creamer | 1 pc. Lindt 95% chocolate | ¼ C peanuts | 3 C lettuce | ¾ C goat yogurt | ¼ C blueberries | ¼ C mushrooms |
| ¼ onion | ¼ C peanuts | ¼ C pecans | 4¼ oz. sardines | 1 French fry | 6–8 oz. almond-coconut milk | 3 oz. salmon |
| 2 C spinach | ½ avocado | 1 slice pepper jack cheese | 14 C goat cheese | ½ tsp chipotle sauce | 2 tbsp heavy whipping cream | 1 C spinach |
| 2 eggs | ¼ Asian pear | 1 slice cheddar cheese | 1 tbsp GF parmesan cheese | ¼ C strawberries | 6 oz salmon | 1 tbsp parmesan |
| 1 slice pepper jack cheese | ¼ C peanuts | ¼ C sauerkraut | 1 tbsp red wine vinegar | ¼ avocado | 1 C zucchini | ¼ C blueberries |
| ¼ C pecans | ¼ C blueberries | 1 tbsp mustard | ¼ C black olives | 1 C green beans raw | 2 C lettuce | 1 C green beans raw |
| ¼ C pecans | 6–8 oz. almond-coconut milk | ¼ C canned tomatoes | ¼ C carrots + couple more | ¼ C strawberries | ||
| ½ chicken breast | ¼ C pecans | 2 tbsp vinegar | 22 cherry tomatoes | |||
| 1 small gluten-free hot dog | 1 C broccoli | |||||
| 1 tbsp GF butter | ||||||
| 2 tbsp grated cheese |
aEntire diet duration was from July 15, 2021 to January 15, 2022.
C, cup; GF, grass fed; LDL-C, low-density lipoprotein cholesterol; pc., piece; tbsp, tablespoon; TC, total cholesterol; tsp, teaspoon.
She returned to her cardiologist 6 months later, and he suggested again that she start medication to lower her LDL-C to 138 mg/dL and her total cholesterol to 229 mg/dL. She was persistent and wanted to keep trying to reduce her cholesterol through diet changes. He recommended that she consult a dietitian, so she underwent a consultation. She did opt to follow a low-cholesterol diet (LCD), which included lower consumption of saturated fats and cholesterol and increased consumption of fiber, nuts, and peanuts (Table 2). Because her diet was not overseen regularly by the registered dietician, she unintentionally lowered her calories and became underweight. Her final lipid panel showed she had lowered her cholesterol level to 138 mg/dL (Figure 1).
Table 2: Low-cholesterol diet (LDL-C 138 mg/dL, TC 229 mg/dL) sample dates March 13, 2022 to March 19, 2022a.
| Sunday | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday |
|---|---|---|---|---|---|---|
| ½ lb. sword fish | ½ C oatmeal | 3/4 C egg whites | 1 beet | 3/4 C egg whites | 3/4 C egg whites | 2 meatballsb |
| ¼ Fuji apple | 2 meatballsb | 6 meatballsb | 1 C brown rice | 2 meatballsb | 2 meatballsb | 1 C spinach |
| ½ tbsp natural peanut butter | 1 pc. Lindt 95% chocolate | ½ C nuts | ¼ C nuts | 4 asparagus | ½ C oatmeal | 6 strawberries |
| Almond paste=¼ C almond flour, 3 tbsp raw cocoa, pinch salt, 1½ tbsp yogurt | ½ tbsp natural peanut butter | 1 pc. Lindt 95% chocolate | 3 meatballsb | ½ C brown rice | ¼ C walnuts | 1½ banana |
| 1½ C corned beef | 4–6 oz. fish | ½ tbsp natural peanut butter | 1 tsp natural peanut butter | ½ C oatmeal | ½ date | 6–8 oz. chocolate milk |
| 1½ C cabbage | ½ C broccoli | 1 small apple | ¼ C walnuts | ¼ C walnuts | 1 avocado | ¼ C mixed nuts |
| ½ C broccoli | 1 apple | 1 carrot | ½ date | ¼ C nuts | 4 oz. chicken | |
| ¼ apple | 4 tbsp natural peanut butter | 2/4 C brown rice | 1 small bag potato chips | 1 pc. celery | 8 oz. cauliflower | |
| 4 meatballsb | 5 meatballsb | 1 avocado | ¼ C almonds | 3 oz. fish | 3 strawberries | |
| 1 tbsp natural peanut butter | 2 C spaghetti squash | Almond paste=¼ C almond flour, 2 tbsp raw cocoa, 1 tbsp raw cocoa, pinch salt, 1½ tbsp yogurt | 3/4 beet | ¼ C pecans | 1 C grapes | |
| 5 meatballsb | Almond paste=¼ C almond flour, 2 tbsp raw cocoa, 1 tbsp raw cocoa, pinch salt, 1½ tbsp yogurt | 1 tbsp natural peanut butter | ||||
| 2 C spaghetti squash |
aEntire diet duration was from March 2, 2022 to August 31, 2022.
bEach meatball consisted of 1/8 C ground turkey plus 1/8 C ground pork.
C, cup; pc., piece; tbsp, tablespoon; tsp, teaspoon.
Family History
Father: High cholesterol and heart attack at the age of 79 years, still living.
Mother: High blood pressure, osteoporosis, still living.
Brother: Healthy, still living.
Discussion
Hyperlipidemia is a metabolic disorder characterized by high levels of blood cholesterol, specifically LDL-C. This is a risk factor for CVD. Cholesterol is introduced into the human body in two ways: it is either consumed in the diet (approximately 20%) or synthesized in the liver (approximately 80%). Cholesterol available for absorption from the gastrointestinal tract includes dietary cholesterol as well as approximately 800–1400 mg/day biliary cholesterol.3 Cholesterol is used for the synthesis of steroid hormones or by the liver to synthesize bile acids, which are essential for the absorption of hydrophobic nutrients. The gall bladder stores the bile acids and releases them into the duodenum and proximal jejunum after the stimulation of cholecystokinin. About 95% of bile acid excreted from the gall bladder is taken back up through the enterohepatic circulation, and the lost acid is replaced through the diet. Cholesterol is important to the transportation of lipoproteins such as LDL-C, high-density lipoprotein cholesterol (HDL-C), and very low-density lipoprotein (VLDL-C).2
The purpose of LDL-C is to deliver lipids and lipid-soluble nutrients to cells. When LDL-C reaches a high level in the blood it is at increased risk of oxidation. This can eventually lead to plaque buildup within the artery walls. Conversely, HDL-C scavenges for excess cholesterol, transporting it back to the liver for disassembly. Therefore, high HDL-C reduces CVD risk. Reduction of LDL-C also corresponds to a reduction of CVD mortality and morbidity. Reducing LDL-C by 10 mmol/L can reduce CVD risk by 22%.2
There are two potential diagnoses for hypercholesterolemia: one is familial hypercholesterolemia (FH) and the other is diet and lifestyle related. FH is a genetic disorder that causes high serum LDL cholesterol levels and early artery disease in both men and women. The most common form of FH, accounting for 80%–90% of cases, is a monogenic, autosomal dominant disorder that causes defects in the gene that encodes the LDL receptor. Higher levels of LDL-C are found in individuals who are homozygotes (or compound heterozygotes) rather than heterozygotes for mutations in this gene. The initial understanding of FH was that cholesterol synthesis within the body increased, but the current understanding is that instead the fractional catabolic rate of LDL is decreased in individuals with LDL receptor gene defects compared with normal individuals, leading to decreased LDL-C clearance. Currently, there are 900 known mutations of the LDL receptor gene.4 Heterozygous FH is not seen in childhood or early adulthood. Homozygous or compound heterozygous FH presents within the first decade of life. Most sufferers have hypercholesterolemia, which includes accelerated atherosclerosis if undiagnosed.2
The protocol for diagnosing FH in the United States includes measuring total cholesterol and LDL-C levels, family history, and physical measurements; however, physical findings are not present in all patients with FH. Genetic testing is part of the screening strategies used in several countries but is infrequent in the United States due to the lack of insurance coverage. The current preventative standard from the AHA/ACC for adults 40–75 years of age with primary ASCVD ranges from clinician-patient discussion before a statin therapy is prescribed to favoring statin therapy initiation if conditions exist that put the patient at a higher risk.1 Patients who are prescribed statins and fibrates complain of various side effects.2
The prevalence of FH varies from 1 in 500 to 1 in 137 depending on the ancestry of the population and the criteria used for defining the disease. A group of researchers used National Health and Nutrition Examination Surveys (NHANES) data from 1999 to 2012 to estimate the prevalence of FH in the United States as 1 in 250, where race and ethnicity played a role in this relatively lower estimated prevalence.5 The researchers used the Dutch Lipid Clinic (DLC) criteria, which classify individuals as definite or probable FH based on LDL-C levels, physical examination findings, genetic criteria, and personal and family history of premature ASCVD. The study excluded individuals if they did not have genetic testing, relevant physical examination findings, a family history of hypercholesterolemia, and a personal history of peripheral arterial disease. The study covered 36,949 noninstitutionalized US adults aged 20 years or over, none of whom were pregnant. For those who reported statin use, a multiplier of 1.43 was applied to LDL-C values to estimate untreated levels. The researchers stated that their inability to assess genetic criteria most likely led to an underestimation of the true prevalence of FH in the US population.
There is more than genetics at play when assessing hypercholesterolemia prevalence. Modern lifestyles are marked by unhealthy diets and sedentary behavior, a combination that puts individuals at risk for increased LDL-C and decreased HDL-C.2
The question is: should individuals not clinically diagnosed with FH, but who have a cholesterol greater than 190 mg/dL, be treated the same as those who are clinically diagnosed with FH? Or should they first be guided to address hyperlipidemia with diet and lifestyle changes? Moreover, should healthcare systems apply a lifestyle rehabilitation protocol prior to medications? Further research is needed to answer these questions.
Dietary Recommendations
Research has uncovered many foods with components that support the body in reducing LDL-C, including phytosterols (Tables 3 and 4),6 beta-glucans, gums, and pectin.2 The consumption of certain plant-based whole foods that have a high affinity for the binding sites of bile acids and compete with cholesterol can reduce cholesterol absorption.3
Table 3: Total phytosterol content in fruits (mg/100 g)6.
| Fruits | Total plant sterols |
|---|---|
| Apple | 13 |
| Banana | 14 |
| Clementine | 16 |
| Fig | 22 |
| Grapefruit | 18 |
| Honeydew melon | 1.8 |
| Kiwi | 9.1 |
| Lemon | 18 |
| Orange | 24 |
| Passion fruit | 44 |
| Peach | 15 |
| Pear | 12 |
| Pineapple | 17 |
| Watermelon | 1.3 |
Table 4: Total phytosterol content in vegetables (mg/100 g)6.
| Vegetable | Total plant sterols |
|---|---|
| Broccoli | 39 |
| Brussels sprouts | 43 |
| Carrot | 16 |
| Cauliflower | 40 |
| Celeriac | 20 |
| Celery | 17 |
| Cabbage, Chinese | 8.5 |
| Cabbage, white | 13 |
| Fennel | 9.8 |
| Kalea | 8.8 |
| Leek | 8.1 |
| Mushrooms | 18 |
| Olives, green | 35 |
| Olives, black | 50 |
| Onion | 8.4 |
| Parsnip | 27 |
| Pepper, green | 7.2 |
| Potatoa | 3.8 |
| Radish | 9.0 |
| Sauerkrauta | 15 |
| Turnip, Swedish | 17 |
| Tomato | 4.7 |
aAnalyzed as eaten, i.e., boiled.
Phytosterols
Plant sterols and plant stanols are commonly known as phytosterols. They are a component of the plant cell membrane with a structure similar to cholesterol, and are found in vegetables (Table 3), fruits (Table 4), nuts, seeds, legumes, and fungi. They inhibit both exogenous cholesterol absorption and endogenous cholesterol formation.7 Although these positive effects were previously thought to taper off at intakes above 2.5 g per day of phytosterol intake, new evidence shows high doses of phytosterols, of about 9 g per day, can achieve a similar effect to drugs that block cholesterol absorption.8 Those who consume a plant-based, whole-food diet ingest higher amounts of phytosterols per day than those who consume a typical Western diet.3 There is no evidence that consumption of phytosterols in whole foods produces negative side effects such as increases in blood pressure or blood coagulation, or deterioration in liver or kidney function.8
Edible Plant Oils
Edible plant oils, if cold-pressed (meaning they are minimally processed to maintain their micronutrient contents) contain squalene, which downregulates hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase activity and reduces cholesterol synthesis.9 They can also be refined, which reduces the value of their micronutrients and other constituents but increases their esterified stanols, which are beneficial in lowering LDL-C.10
Nuts
Nuts contain fatty acids, fibers, proteins, minerals, vitamins, carotenoids, and phytosterols with potential antioxidant actions. They are frequently associated with a reduction of chronic disease risk, especially diabetes and CVD.11
Almonds improve LDL-C when consumed in amounts between 42 and 75 g per day, especially when added to a cholesterol-lowering diet. For individuals who do not choose to take or cannot use statins, almonds may be helpful. Weight gain was not observed with high amounts of almonds.11
Walnuts improve LDL-C, total cholesterol, and triglyceride levels, as well as overall cardiovascular risk, when consumed in amounts between 40 and 65 g per day. Those who consumed a Mediterranean diet and replaced 40% of the fat with walnuts had a reduction in LDL-C of 7.3%.11
For individuals with an increased risk of CVD, walnut consumption resulted in higher activity of paraoxonase, a protein that has protective cardiovascular properties; enhanced endothelial function; improved adiponectin concentration; and improved Apo A concentrations. Weight gain was not observed with high amounts of walnuts.11
Brazil nuts and cashews are beneficial to the cardiovascular system. Brazil nuts improve selenium status, which in turn increases the activity of glutathione peroxidase, an important antioxidant. Individuals with hypertension and dyslipidemia who consume 13 g/day of Brazil nuts have reduced oxidative stress, reduced oxidized LDL-C, lower blood pressure, and increased antioxidant status. Those with hypercholesterolemia who consumed between 28 and 64 g/day of cashews had a decrease in total cholesterol and LDL-C.11
Soluble Fibers
Soluble fibers from whole foods can attract water in the intestine and form a viscous matrix that reduces serum cholesterol absorption. Soluble fibers include beta-glucans, pectin, and gums.2
Beta-glucans are a group of polysaccharides that are fermented by the microbiota in the intestine and produce short-chain fatty acids.12 Beta-glucan-rich foods include oat bran, rolled oats, whole oat flour, whole grain barley, hull-less whole grain barley, seaweed (red, brown, green, and their polysaccharide extracts), and several types of edible mushrooms.13 Barley has the highest functional value among cereal crops due to its high amounts of beta-glucans, low glycemic index, antioxidant properties, and resistant starch. Black hull-less and blue hull-less barley varieties are especially high in beta-glucans.12 Consuming a meal that contains 5 g of oat beta-glucans was shown to lower the absorption of cholesterol.2
Gums are complex carbohydrates that form gels. Konjac is a gum formed into a food source that comes from the konjac plant (Amorphophallus konjac) grown in Asia. The plant has a starchy corm from which the extract glucomannan is derived. A study of 22 individuals taking 1500 mg of glucomannan combined with exercise (ranging from no exercise to endurance exercise) showed a reduction in body mass, percentage fat mass, total cholesterol, and LDL-C.14 In addition, when added to a typical Western diet, research shows glucomannan also reduces triglycerides, free fatty acids, liver cholesterol, and non-HDL-C.2 Konjac is available as rice, pasta, and gelatin.
Pectin is present in the cell wall and middle lamella of fruits and vegetables2 and has gel-forming and binding properties.3 When pectin-containing foods ripen, the degradation of the plant produces polygalacturonic acid (pectic acid), which has been shown to statistically significantly lower liver cholesterol. Combinations of pectin molecules have been examined and compared with lovastatin.2 Three different combinations of pectin were administered in an animal model of familial hypercholesterolemia; these combinations included pectin and polyphenols, pectin and phytosterols, and pectin with polyphenols and phytosterols. Amounts included 30 g/day of pectin; 6 g/day of polysterols from nuts, seeds, and oils; and 20 g/day of polyphenols. It was found that the combination of pectin, phytosterol, and polyphenol reduced LDL-C levels and was half as efficacious as lovastatin in reducing total cholesterol. Another combination of pectin (as apple) and pea protein has been shown to effectively reduce plasma cholesterol by upregulating the hepatic cholesterol turnover genes.2 Apple, apricot, banana, peach, plum, papaya, mango, tomato,15 and pea protein2 are foods rich in pectin.
Conclusion
Nutrition should be evaluated as a treatment for hypercholesterolemia in two ways: as an alternative to traditional treatment or as an adjunct to existing treatment. Either way, it should always be in the treatment plan. If the patient in our case report had regularly seen a registered dietitian or certified nutrition professional to correct her diet, she may have lowered her cholesterol levels within the first 6 months (Table 1). The evidence clearly shows this was a possibility, since she was able to lower her cholesterol within the second 6-month diet period (Table 2) after a considerable increase in LDL-C. Lowering cholesterol levels quickly reduces arterial oxidation damage and fat deposits. Foods that contain phytosterols, beta-glucans, pectin, and soluble fibers should be a part of a diet aimed at lowering LDL-C and consistently consumed in the daily diet for protective benefits.
Differences were found in the diet logs between the HCD and the LCD, which may have been the catalyst for the lowered LDL-C (Tables 1 and 2). Comparing the two diets, the LCD had fewer calories, and the patient became underweight. A 5%–10% weight loss has been shown to reduce cholesterol levels, so this may have contributed to her lowered cholesterol.16 While she was on the HCD she consumed an average of 12 eggs per week, and five servings of beef/sausage/or bacon per week, which could account for the increase in her cholesterol blood serum. During the LCD, whole eggs were replaced with egg whites, the egg white consumption was reduced to 4.7 egg whites per week, and beef/sausage/or bacon consumption was reduced to 1.1 servings per week. In the LCD, fish consumption went up by 56% per week on average. The number of daily servings of nuts and natural peanut butter (≥36 g/serving) increased in the LCD. The number of days on which she consumed nuts per week also increased. Maintaining concentrations of phytosterols in the plasma may have added to the cholesterol-lowering benefits.
This individual reduced her LDL-C by approximately 50% in a 6-month period. From this result we conclude that some individuals with moderately high cholesterol levels may be able to reduce them through dietary changes alone and thus be able to forgo cholesterol-lowering drugs and their side effects. The analysis of her diet suggests the lowered saturated and cholesterol fats, increased healthy fats, consistent intake of phytosterol foods, and weight loss were likely contributors to her lowered cholesterol.
References
- American College of Cardiology. 2018 AHA/ACC Multisociety Guideline on the Management of Blood Cholesterol. American College of Cardiology. https://www.acc.org/latest-in-cardiology/ten-points-to-remember/2018/11/09/14/28/2018-guideline-on-management-of-blood-cholesterol. Accessed May 5, 2023.
- Nie Y, Luo F. Dietary fiber: an opportunity for a global control of hyperlipidemia. Oxid Med Cell Longev. 2021;2021:5542342. https://doi.org/10.1155/2021/5542342.
- Jesch ED, Carr TP. Food ingredients that inhibit cholesterol absorption. Prev Nutr Food Sci. 2017;22(2):67. https://doi.org/10.3746/PNF.2017.22.2.67.
- Bouhairie VE, Goldberg AC. Familial hypercholesterolemia. Cardiol Clin. 2015;33(2):169–79. https://doi.org/10.1016/J.CCL.2015.01.001.
- De Ferranti SD, Rodday AM, Mendelson MM, et al. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States national health and nutrition examination surveys (NHANES). Circulation. 2016;133(11):1067–72. https://doi.org/10.1161/CIRCULATIONAHA.115.018791.
- Normén L, Johnsson M, Andersson H, Van Gameren Y, Dutta P. Plant sterols in vegetables and fruits commonly consumed in Sweden. Eur J Nutr. 1999;38(2):84–9. https://doi.org/10.1007/S003940050048.
- Normén L, Ellegård L, Brants H, et al. A phytosterol database: fatty foods consumed in Sweden and the Netherlands. J Food Compost Anal. 2007;20(3–4):193–201. https://doi.org/10.1016/J.JFCA.2006.06.002.
- Laitinen K, Gylling H. Dose-dependent LDL-cholesterol lowering effect by plant stanol ester consumption: clinical evidence. Lipids Health Dis. 2012;11:140. https://doi.org/10.1186/1476-511X-11-140.
- Bester D, Esterhuyse AJ, Truter EJ, Van Rooyen J. Cardiovascular effects of edible oils: a comparison between four popular edible oils. Nutr Res Rev. 2010;23:334–8. https://doi.org/10.1017/S0954422410000223.
- Phillips KM, Ruggio DM, Toivo JI, et al. Free and esterified sterol composition of edible oils and fats. J Food Compost Anal. 2002;15(2):123–42. https://doi.org/10.1006/JFCA.2001.1044.
- De Souza RGM, Schincaglia RM, Pimente GD, Mota JF. Nuts and human health outcomes: a systematic review. Nutrients. 2017;9(12):1311. https://doi.org/10.3390/NU9121311.
- Zeng Y, Pu X, Du J, et al. Molecular mechanism of functional ingredients in barley to combat human chronic diseases. Oxid Med Cell Longev. 2020;2020:3836172. https://doi.org/10.1155/2020/3836172.
- Mirończuk-Chodakowska I, Kujawowicz K, Witkowska AM. Beta-glucans from fungi: biological and health-promoting potential in the COVID-19 pandemic era. Nutrients. 2021;13(11):3960. https://doi.org/10.3390/NU13113960.
- Devaraj RD, Koteswara Reddy C, Xu B. Health-promoting effects of konjac glucomannan and its practical applications: a critical review. Int J Biol Macromol. 2019;126:273–81. https://doi.org/10.1016/j.ijbiomac.2018.12.203.
- Food Science and Processing: Post-harvest changes and storage. n.d. Available at http://ecoursesonline.iasri.res.in/mod/page/view.php?id=111787. Accessed April 23, 2023.
- Brown JD, Buscemi J, Milsom V, et al. Effects on cardiovascular risk factors of weight losses limited to 5–10%. Transl Behav Med. 2016;6:339–46. https://doi.org/10.1007/s13142-015-0353-9.