Abstract
The current standard-of-care treatment regimens for cancer frequently have serious and irreversible adverse effects. Ideally, therapeutic modalities should help control symptoms and improve the patient's quality of life while causing minimal or no toxic effects. In this regard, it is worth examining cannabidiol (CBD) for its potential anticancer properties. CBD may possess antitumor activity through several mechanisms, including regulating reactive oxygen species, endoplasmic reticulum stress, inflammation, and immune modulation. In addition, pre-clinical studies indicate that CBD is a potential modulator of growth factors and induces apoptosis in tumor cells. This review summarizes the evidence regarding the effects of CBD as a non-toxic adjuvant in cancer care.
Introduction
Cancer is one of the leading causes of death worldwide and the first or second leading cause in people younger than 70 in 112 countries.1 Lifestyle habits associated with increased cancer risk, such as tobacco, obesity, low physical activity, alcohol, processed food, and other dietary/nutritional issues, have become more prevalent and may have led to an increased incidence of this condition.
Limitations of ineffective treatment options for cancer that have few side effects provide a challenge to both physicians and patients. Chemotherapy is a non-targeted treatment that damages both healthy and malignant cells. In addition to significant failure rates, studies show that 80% of patients experience nausea, hair loss, and fatigue during their chemotherapy regimen.2 In one study, 46% of patients considered stopping treatment by the sixth cycle of chemotherapy. Patients treated with high-dose cyclophosphamide, thiotepa, and carboplatin (CTC) chemotherapy have a greater decline in cognitive performance than patients in standard-dose 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) chemotherapy when compared with healthy controls.3 A variety of outcomes, such as quality of life, cancer cell death, tumor size, and survival, have been evaluated to determine if adjunctive treatment based on food and other natural products can reduce toxicity and improve the efficacy of current therapies.4,5 Some adjunctive natural treatments such as acupuncture, yoga, mindfulness, high-dose vitamin C, and herbals have been associated with improved quality of life6–8 and possibly contribute to prolonged survival.9
The authors support the notion that cancer originates mostly from disturbances in energy production10 and that the genetic changes observed with cancer originate from these metabolic derangements.11 Over the last decades, pre-clinical studies suggest a role for cannabidiol (CBD) in regulating biological processes of mitochondria such as respiration and bioenergetics, epigenetics, apoptosis, mitochondrial fission, fusion, and biogenesis.12 Despite gaps in our knowledge about the magnitude and direction of these effects, emerging data support the notion that CBD modulates mitochondrial function and morphology in a dose-dependent way, inducing oxidative stress at higher CBD concentrations.13
The Endocannabinoid System
The endocannabinoid system (ECS) is present in all mammalian species and is thought to be a crucial modulator of homeostasis. The ECS is an extensive network of cannabinoid molecules, enzymes, and receptors produced on demand in the central nervous system. It works retrograde to modulate several processes, including neuronal differentiation and migration and cognitive and physiological processes.14 Furthermore, at a systemic level, the ECS regulates aspects of various physiological, behavioral, immunological, and metabolic functions.15 The CB1 receptors are by far the most abundant G-protein coupled receptors in the central nervous system, and there are several endocannabinoids in the brain. The first endocannabinoid discovered was anandamide. The CB2 receptors are predominantly expressed in immune cells where activation results in anti-inflammatory and antispasmodic action in the intestines and immunosuppressant function.16
In addition to CB1 and CB2, cannabinoids can act through transient receptor potential (TRP) channels and peroxisome proliferator-activated receptors (PPARs).
The ECS influences the nervous and immune systems, mitochondrial energy, and other metabolic processes. The modulation of metabolic pathways and/or agonism or antagonism of the receptors of the ECS offer promising opportunities for developing novel therapeutic options for various conditions. Research regarding the components of the ECS and various cannabinoids is very active and promising in diseases such as mood and anxiety disorders, pain management, inflammation, cardiovascular disorders, diabetes, stroke, neurological and neurodegenerative disorders, schizophrenia, epilepsy, autoimmune diseases, and cancer.17
The ECS is altered in several types of cancers, including breast, glioma, leukemia, and lung cancer. Furthermore, these alterations have been associated with prognosis and outcome,18 implying that ECS may have a role in tumor growth and progression since CBD may exert anticancer activity by inhibiting the proliferation, angiogenesis migration, and invasion19 of cancer cells. Thus, ECS's potential to affect cancer outcomes needs to be studied further to ascertain more fully.
Phytocannabinoids
Phytocannabinoids are natural terpenoids or phenolic compounds derived from Cannabis sativa that can interact with the ECS.20 Cannabis has generally been considered a safe and effective therapeutic botanical remedy since ancient times.21 Between 1840 and 1900, the European and American Journal of Medicine and Science published over 100 articles on the therapeutic use of cannabis. However, federal legislation that outlawed sales of alcohol from 1920 to 1933 was followed by other federal laws that initially taxed (1937) and subsequently classified cannabis as a controlled substance since the approval of the Federal Controlled Substance Act (CSA) in 1970. Cannabis was included in the American Pharmacopeia until 1942. In 1964, the structure of tetrahydrocannabinol (THC) was determined in Israel by Mechoulam and Gaoni.22 Decades later, in the United States, several states approved local legislation to allow the medical use of cannabis. Currently, the medical use of cannabis is legal in 36 states. In 2018 the Farm Bill was approved, removing hemp from the definition of marijuana in the CSA.23 Cannabis is also available by prescription in Canada, the Netherlands, Israel, and Germany.19,20,24 The European Union (EU) recently proclaimed that all EU citizens must be granted access to medical cannabis within the next 4 years.25
CBD is the second most common phytocannabinoid in cannabis and the most common in the hemp plant. The CBD molecule is shown with a tetrahydro-biphenyl skeleton formed by the monoterpene, p-cymene (Figure 1). CBD can be converted into Δ9-THC (THC) via an acid-catalyzed reaction.26
Figure 1: Cannabinoids and terpenes.
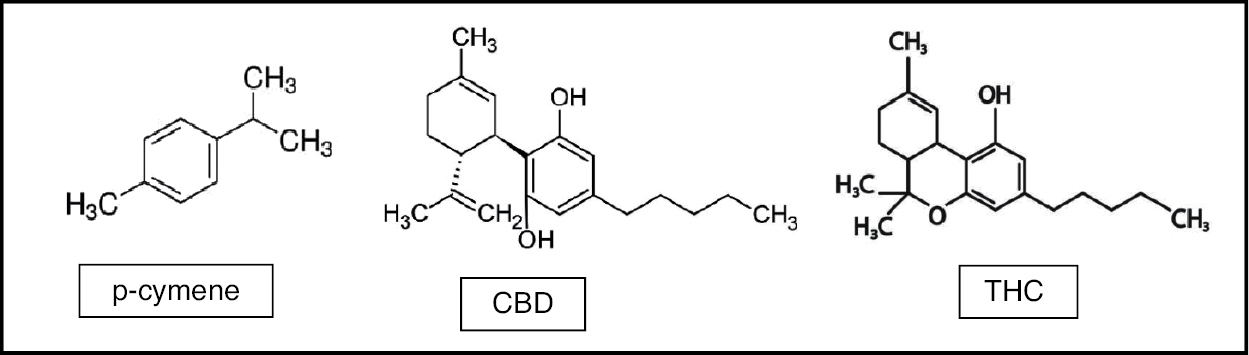
CBD does not necessarily act through the cannabinoid type 1 (CB1) receptor but has many other receptors and action sites.27 CBD has reduced binding affinity for either CB1 or CB2 receptors, but it can antagonize them in the presence of THC.28
CBD has been shown to modulate the tumor microenvironment, reducing the secretion of cytokines from cancer cells. Decreased recruitment of macrophages from the tumor microenvironment by cancer cells suppresses angiogenesis within the tumor, limiting the supply of oxygen and nutrients needed for tumor growth.29 In vitro and in vivo models support multiple antiangiogenic mechanisms associated with downregulating several angiogenesis-related molecules by broadly influencing several pathways involved in this process. These include modification of the expression pattern of angiogenesis-related proteins, inhibition of endothelial morphogenesis, and outgrowth of capillary-like structures. Its double action on both tumor and endothelial cells suggests that CBD could be an effective cancer therapy agent.30
CBD, by itself or with other agents, has been found to induce cell death, inhibit cell migration and invasion in vitro, reduce tumor size, vascularization, growth, and weight, and increase survival and stimulate tumor regression in vivo.31–38 CBD may have multitargeted actions in cancer, such as antiproliferative, pro-apoptotic, cytotoxic, anti-invasive, anti-antiangiogenic, anti-inflammatory, and immunomodulatory effects.39,40 In addition, a case series showed increased median survival in glioblastoma patients using CBD,41 and a systematic review concluded that CBD is relatively safe.42
CBD Pharmacokinetics
The pharmacokinetics of CBD depending on the method of administration.
The primary route used in controlled human studies is oral administration in doses ranging from 20 to 6,000 mg. The mean time to maximum concentration (Tmax) for oral administration ranged from 1 to 6.13 h post-ingestion43 because of inter-individual variations in metabolizing enzymes.
Epidiolex® is a CBD isolate product undergoing meticulous pharmacokinetic evaluation and testing in clinical trials.43 The US FDA approved it for the treatment of intractable childhood-onset seizures. One study evaluated the pharmacokinetics of oral CBD in healthy adults and found that a single administration of Epidiolex® had a Tmax range of 3–5 h.44 The half-life (t½) of CBD (Epidiolex®) after a single dose has been reported to be 10–17 h.44 However, in children with chronic dosing for treatment-resistant epilepsy, the half-life of CBD (Epidiolex) was found to be between 21.6 and 33.5 h.45
The half-life for an oromucosal spray is 1.4–10.9 h; for intravenous administration, 24 h; and for oral administration, 2–5 days.46 Despite the substantial therapeutic potential of CBD, its development by the pharmaceutical industry as an effective drug needs to be improved by inherent attributes of its chemistry, such as low bioavailability, low water solubility, and irregular pharmacokinetic profiles.46 CBD is generally consumed in over-the-counter products that are of undetermined composition.
CBD products can be classified into three main groups: (1) CBD isolate, the purified form of CBD; (2) full-spectrum hemp extracts containing CBD and other naturally occurring phytocompounds in the cannabis plant, such as terpenes and other cannabinoids (by legal definition, full-spectrum hemp extracts cannot contain more than 0.3% THC); (3) broad-spectrum CBD, which is similar to full spectrum, but lacks THC.47,48
Extensive research has been published about the potential therapeutic value of cannabis and its components in cancer.21 Important work has also been done about the entourage effect, whereby the multiple naturally occurring components of the whole plant, including cannabinoids, terpenoids, and flavonoids, work synergistically to produce unique effects and benefits.49,50 However, the entourage effect is a phenomenon that requires CBD products derived from full-spectrum hemp (or cannabis) and is not a feature of CBD isolates.
Various formulations, such as self-emulsifying drug delivery systems, improved crystal formulations, and other solid-state delivery formulations, are being explored to improve the reliability of the intended effect.46 However, at this time, it is too early to determine a clear advantage of any dosage delivery form in managing cancer.
Effect of CBD on Tumorigenesis
The potential antitumor activity of CBD relates to its regulation of reactive oxygen species (ROS), endoplasmic reticulum (ER) stress, inflammation, and immune modulation.51 CBD is occasionally used in palliative care in oncology patients to stimulate appetite and reduce pain and nausea associated with cancer treatment. However, the evidence of the level of efficacy in the variety of reported benefits needs to be further investigated in this area, and active research is currently underway.52
Pre-clinical studies suggest CBD possesses antiproliferative and pro-apoptotic effects and inhibits cancer cell migration, adhesion, and invasion. Recent evidence regarding the efficacy of CBD in the modulation of tumorigenesis in several types of cancer has demonstrated important in vitro and in vivo effects on tumor cell proliferation, viability, and invasion; ROS production; and molecular cell signaling in breast, glioma, leukemia, lung thyroid, and colon cancer models.53 In addition, CBD has been found to downregulate the expression of the pro-metastatic gene inhibitor.54 Also, in vitro studies revealed that CBD inhibits angiogenesis by multiple mechanisms, showing effects on migration, invasion, sprouting, and angiogenesis.55
CBD is thought to influence the ECS by inhibiting fatty acid amide hydrolase (FAAH), the catabolizing enzyme for the endocannabinoid anandamide.56 CBD may also antagonize CB1/CB2 agonists at the nanomolar range.57 In addition, there is evidence that CBD also activates 5-HT1A/2A/3A serotonergic and vanilloid receptors, antagonizes alpha-1 adrenergic and μ-opioid receptors, and inhibits synaptic uptake of noradrenaline, dopamine, serotonin, and gamma-aminobutyric acid (GABA).58–61 Other studies indicate that it affects mitochondrial activities, including calcium influx.
There are many in vitro and in vivo studies supporting the anticancer effects of CBD, and also clinical studies in glioma, breast cancer, colorectal cancer, leukemia/lymphoma, prostate cancer, and other cancers, which have been summarized by Seltzer et al.51
CBD and Mitochondrial Regulation
CBD and other cannabinoids exert various biological effects in the mitochondria by receptor- and non-receptor-mediated mechanisms. The effects on the mitochondria can be related to alterations in the mitochondrial membranes or an indirect effect on the cell membrane CB1.62 Most cancer cells use aerobic glycolysis. Aerobic glycolysis increases glucose uptake and lactic acid production and drives the expression of gene profiles necessary for glycolysis.63 Aerobic respiration or oxidative phosphorylation (Krebs cycle and electron transport system), which occurs in the mitochondria, is much more efficient and produces more energy than anaerobic respiration (glycolysis) in the cytoplasm. The energy surplus produced by the mitochondria provides the necessary energy to promote and sustain the differentiated state.
Mitochondrial dysfunction may contribute to malignant transformation via three mechanisms: (1) increased mitochondrial ROS production64; (2) abnormal accumulation of mitochondrial metabolites such as fumarate, succinate, and 2-hydroxyglutarate65; and (3) functional deficits in mitochondrial outer membrane permeabilization (MOMP) that allow for the survival of malignant precursors.44 Increased ROS favors the accumulation of potentially oncogenic DNA defects and activation of potentially oncogenic signaling pathways. In addition, malignant cells usually require defects in MOMP to evade regulated cell death (RCD).66
A recent study demonstrated that CBD could attenuate rodent oxygen-glucose deprivation and reperfusion (OGD/R) by improving mitochondrial energy metabolism and normalizing hypoxia-induced oxidative stress.67 CBD supplementation was studied using a hippocampal neuronal cell line and showed damage attenuation by oxygen-glucose-deprivation/reperfusion (OGD/R)-induced cell death. CBD also attenuated intracellular ROS generation and lipid peroxidation, reversing the abnormal changes in antioxidant biomarkers.68
There are some studies on the effect of CBD on mitochondrial function leading to anticancer effects that show promising results. One study used cultured gastrointestinal cancer cells to observe the potential modulating effect on mitochondrial respiration. Administration of 4 μM CBD reduced basal respiration rate and ATP production in tumor cells.12 In addition, CBD decreased cancer proliferation in mouse tumor cells. Other in vitro studies have demonstrated that CBD treatment leads to a biphasic increase in intracellular calcium levels and changes in mitochondrial function and morphology, resulting in cell death. The molecular target of CBD for this effect is the mitochondrial voltage-dependent anion channel 1 (VDAC1).69
CBD and Cancer Gene Expression
Cancer cells overexpress multiple genes that are involved in growth and proliferation. For this reason, modulation of gene expression in tumor cells can decrease proliferation and tumor growth. Studies suggest that CBD can modulate gene expression. ID-1, a helix-loop-helix protein that plays a prominent role in tumor cell proliferation and progression, is one of the most extensively studied transcription factors associated with tumor cells.70 ID-1 is important in endothelial cell proliferation and indicates that ID-1 represses p16 expression, resulting in delayed senescence, which may have implications for developing endothelial-derived tumors.71 In addition, western blot analysis of ID-1 was performed in 15 pairs of gastric cancer tissues compared to adjacent tissue. ID-1 was more expressed in 11/15 cancer tissues than in adjacent tissues. It was concluded that the ID-1 protein might play an important role in gastric carcinogenesis, and high-level ID-1 expression may be associated with the malignant potential of tumor cells.72
Functional studies have shown that ID-1 is required for tumor-initiating functions that lead to metastases in the lung.70 Downregulation of ID-1 in vivo cells inhibited tumor progression.54 ID-1 protein is overexpressed in more than 20 types of cancer and can promote tumorigenesis in a broad range of tissues. In addition, ID-1 expression protects cells from apoptosis during chemotherapy by regulating the Raf-1/MAPK and JNK pathways.73
Multiple studies have shown that CBD downregulates the promoter sequence of the ID-1 gene, inhibiting the production of ID-1 protein.54,74 It has been theorized that a decrease in ID-1 protein leads to the downregulation of growth-promoting and invasion-promoting genes. CBD is, therefore, the first non-toxic agent shown to have a downregulating effect on ID-1 expression.74 CBD has also been shown to upregulate the expression of GDF15 (growth differentiation factor 15), a cytokine associated with tissue differentiation, and to inhibit the expression of a transcriptional activator involved in cell proliferation that is upregulated in several types of cancer. These findings suggest that CBD can modulate the proliferation of cancer cells.54
CBD and Growth Factors
For a tumor to grow, it needs to generate new blood vessels. Growth factors that facilitate angiogenesis, therefore, may promote tumor progression. CBD has been shown in an animal model to reduce breast cancer growth by inhibiting epidermal growth factor (EGF).75 Epidermal growth-factor receptor (EGFR), a growth-factor receptor for a tyrosine kinase, is commonly overexpressed in multiple cancer types and promotes tumor growth.76 A study of gene expression in non-small cell lung cancer patients found that THC and CBD inhibited the expression of EGFR in lung cancer cells, potentially reducing metastasis.77
CBD and Cancer Cell Apoptosis
TP53 is a tumor suppressor gene that is heavily involved in cell cycle regulation.78,79 When p53 detects cellular DNA damage, it terminates the cell cycle and stimulates apoptosis.78 Loss of p53 function results in uncontrolled cell proliferation and tumor cell resistance to apoptosis. CBD has been shown to inhibit human lung cancer cells. CBD treatment upregulates apoptosis-related proteins, such as p53, and several others, indicating that CBD regulates several types of cell death.80
Tumor cell studies with various concentrations of CBD resulted in the reduction of viable tumor cells.81 Similar results were found in an animal model where CBD caused a dose-dependent decrease in viable tumor cells. CBD was found to induce apoptosis in tumor cells in the gastric epithelial lining but spared normal gastric epithelial cells.82 CBD has been found to increase the generation of ROS and facilitate apoptosis and autophagy. The intricate interplay between apoptosis and autophagy in CBD-treated breast cancer cells plays an important role in its antineoplastic effects.83
CBD and Exosomes
Exosomes are cup-shaped microvesicles that mediate cellular communication.84,85 Interestingly, the volume of exosomes released by tumor cells is higher than in normal cells. Tumor cells use exosomes to share proteins and genetic material contributing to their malignancy and growth, including a protein that counteracts the effects of cancer treatment. These exosomes also interact with the immune system to shield cancer cells from detection.86 Studies have shown that CBD reduces the number of exosome microvesicles containing specific pro-oncogenic proteins and increases those containing anti-oncogenic proteins. It has been proposed that the anticancer effects of CBD are partly due to its regulatory effects on the biogenesis of exosome microvesicles. The modulating effects of CBD on exosome release were found to be dose-dependent and cancer cell type-specific. Mitochondrial function and protein expression changes may be responsible for sensitizing cancer cells to chemotherapy.87 These effects were greater with CBD than with the antineoplastic drug temozolomide used to treat glioblastoma.88
Studies have shown that, at concentrations of 5 μM, CBD can block 50% of exosome release in some prostate cancer, hepatocellular carcinoma, and breast adenocarcinoma cell lines.87,88 These concentrations are clinically achievable when using higher oral doses. A phase I randomized placebo-controlled dose escalation study evaluated three dose levels (5 mg/kg, 10 mg/kg, and 20 mg/kg) of CBD. The highest dose achieved plasma concentrations of 1,100 ng/mL, equivalent to 3.5 μM.89 Another pharmacokinetic study with human subjects demonstrated that oral administration could achieve or exceed these concentrations. The use of CBD doses of 750 to 1500 mg was tolerated. Some patients experienced mild adverse effects but reported no severe adverse effects.44
The inhibition of exosomes by CBD is selective to the membrane of cancer cells. A study that investigated the synergistic effect of a mixture of different cannabinoids, including THC, cannabigerol (CBG), cannabinol (CBN), and CBD on human breast cancer cell lines found that the combination produced cell cycle arrest followed by the induction of apoptosis without producing adverse effects in normal cells.90 CBD inhibition of exosome release is dose-dependent. Effects of inhibiting exosome release varied by cancer type, but the net effect was that CBD led to slower cancer growth and increased susceptibility to cancer treatment.91,92 Compared to other exosome inhibitors, CBD had a stronger inhibitory effect in some cancers than even the most potent pharmaceutical exosome inhibitors.87
CBD and Chemotherapy
Because CBD has low aqueous solubility and is highly sensitive to the effects of light, temperature, and oxidation,93,94 it is challenging to design formulations that enable its use along with chemotherapy regimens. Microencapsulation and nanoparticles have been examined to overcome these challenges. Microencapsulation also allows for the prolongation of antitumor activity after a single administration.
An in vitro study examined the effects of CBD-enriched microparticles and chemotherapy on estrogen receptor-positive and estrogen, progesterone, and HER-2 receptor-negative (triple-negative) breast cancer cells. Combining CBD with paclitaxel (PTX) or doxorubicin (DOX) heightened the effects of the antineoplastic agents on all cancer cell types, potentially allowing for a lower dose of chemotherapy. The study concluded that CBD-containing microparticles had a synergistic effect with both PTX and DOX.95
In another experimental study, CBD microparticles used as a monotherapy reduced ovarian antitumor activity for at least 10 days, showing an approximate 1.5-fold decrease in tumor growth compared to untreated cells.96 The combination of PTX and 10 μM of CBD showed an 8- to 10-fold decrease in PTX IC50 (half maximal inhibitory concentration). PTX treatment alone reduced tumor cell growth 1.5-fold, but in combination with daily CBD and a one-time administration of CBD monotherapy, tumor growth inhibition was 2-fold that of untreated cells. Pre-clinical research has shown that a polymeric nanoparticle form of CBD had better antiproliferative activity in ovarian cancer cells than a CBD solution.97 Other studies have similar outcomes in glioblastoma cells, which showed increased sensitivity to chemotherapeutic agents (carmustine, temozolomide, doxorubicin, and cisplatin) with the use of CBD.98,99
Conclusion
CBD may have analgesic, antinausea, and anxiolytic properties that could be useful to cancer treatment patients. Pre-clinical studies suggest CBD may also have anticancer activity through various mechanisms. It has been found to inhibit the release of exosomes from tumor cells that contribute to their malignancy and growth. In addition, CBD can be used to sensitize cancer cells to chemotherapy. Studies have also shown that CBD may lower the toxicity of some cancer chemotherapeutic compounds and may have a synergistic effect when combined with chemotherapy.
Considering that most currently available treatment options for cancer are invasive and have harsh adverse effects, it must be a priority that we examine new, safer alternatives to enhance conventional cancer care. The pre-clinical evidence for CBD in cancer warrants further clinical trials. Some prospective randomized trials are underway.100
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
- Love RR, Leventhal H, Easterling DV, Nerenz DR. Side effects and emotional distress during cancer chemotherapy. Cancer. 1989;63(3):604–12.
- Schagen SB, Muller MJ, Boogerd W, et al. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. J Natl Cancer Inst. 2006;98(23):1742–5. https://doi.org/10.1093/jnci/djj470.
- Thomsen M, Vitetta L. Adjunctive treatments for the prevention of chemotherapy- and radiotherapy-induced mucositis. Integr Cancer Ther. 2018;17(4):1027–47. https://doi.org/10.1177/1534735418794885.
- Li SG, Chen HY, Ou-Yang CS, et al. The efficacy of Chinese herbal medicine as adjunctive therapy for advanced non-small cell lung cancer: a systematic review and meta-analysis. PLoS One. 2013;8(2):e57604. https://doi.org/10.1371/journal.pone.0057604.
- Sasaki Y, Cheon C, Motoo Y, et al. [Complementary and Alternative Medicine for Breast Cancer Patients: An Overview of Systematic Reviews]. Yakugaku Zasshi. 2019;139(7):1027–46. Japanese. https://doi.org/10.1248/yakushi.18-00215. PMID: 31257250.
- Vollbracht C, Schneider B, Leendert V, et al. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: results of a retrospective, multicentre, epidemiological cohort study in Germany. In Vivo. 2011 Nov-Dec;25(6):983–90. PMID: 22021693.
- Bar-Lev Schleider L, Mechoulam R, Sikorin I, et al. Adherence, safety, and effectiveness of medical cannabis and epidemiological characteristics of the patient population: a prospective study. Front Med (Lausanne). 2022;9:827849. https://doi.org/10.3389/fmed.2022.827849.
- Linus-Lojikip S, Subramaniam V, Lim WY, Hss AS. Survival of patients with advanced and recurrent ovarian cancer treated using integrative medicine in Malaysia: a case series. Complement Ther Clin Pract. 2019;37:73–85. https://doi.org/10.1016/j.ctcp.2019.09.001.
- Gonzalez MJ, Miranda Massari JR, Duconge J, et al. The bio-energetic theory of carcinogenesis. Med Hypotheses. 2012;79(4):433–9. https://doi.org/10.1016/j.mehy.2012.06.015.
- Seyfried TN, Flores RE, Poff AM, D'Agostino DP. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis. 2014;35(3)515–27. https://doi.org/10.1093/carcin/bgt480.
- Chan JZ, Duncan RE. Regulatory effects of cannabidiol on mitochondrial functions: a review. Cells. 2021;10(5):1251. https://doi.org/10.3390/cells10051251.
- Mould RR, Botchway SW, Parkinson JRC, et al. Cannabidiol modulates mitochondrial redox and dynamics in MCF7 cancer cells: a study using fluorescence lifetime imaging microscopy of NAD(P)H. Front Mol Biosci. 2021;8:630107. https://doi.org/10.3389/fmolb.2021.630107.
- Lu HC, Mackie K. Review of the endocannabinoid system. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6(6):607–15. https://doi.org/10.1016/j.bpsc.2020.07.016.
- Joshi N, Onaivi ES. Endocannabinoid system components: overview and tissue distribution. Adv Exp Med Biol. 2019;1162:1–12. https://doi.org/10.1007/978-3-030-21737-2_1.
- Kendall DA, Yudowski GA. Cannabinoid receptors in the central nervous system: their signaling and roles in disease. Front Cell Neurosci. 2017;10:294. https://doi.org/10.3389/fncel.2016.00294.
- Lowe H, Toyang N, Steele B, et al. The endocannabinoid system: a potential target for the treatment of various diseases. Int J Mol Sci. 2021;22(17):9472. https://doi.org/10.3390/ijms22179472.
- Tomko AM, Whynot EG, Ellis LD, Dupré DJ. Anti-cancer potential of cannabinoids, terpenes, and flavonoids present in cannabis. Cancers (Basel). 2020;12(7):1985.
- Fraguas-Sánchez AI, Martín-Sabroso C, Torres-Suárez AI. Insights into the effects of the endocannabinoid system in cancer: a review. Br J Pharmacol. 2018;175(13):2566–80. https://doi.org/10.1111/bph.14331.
- Gonçalves ECD, Baldasso GM, Bicca MA, et al. Terpenoids, cannabimimetic ligands, beyond the cannabis plant. Molecules (Basel, Switzerland). 2020;25(7):1567. https://doi.org/10.3390/molecules25071567.
- Afrin F, Chi M, Eamens AL, et al. Can hemp help? Low-THC cannabis and non-THC cannabinoids for the treatment of cancer. Cancers (Basel). 2020;12(4):1033. https://doi.org/10.3390/cancers12041033.
- Crocq MA. History of cannabis and the endocannabinoid system. Dialogues Clin Neurosci. 2020;22(3):223–8. https://doi.org/10.31887/DCNS.2020.22.3/mcrocq.
- Alharbi YN. Current legal status of medical marijuana and cannabidiol in the United States. Epilepsy Behav. 2020;112:107452. https://doi.org/10.1016/j.yebeh.2020.107452.
- Aggarwal SK, Carter GT, Sullivan MD, et al. Medicinal use of cannabis in the United States: historical perspectives, current trends, and future directions. J Opioid Manag. 2009;5(3):153–68. https://doi.org/10.5055/jom.2009.0016.
- Roychoudhury P, Kapoor AK, Walsh D, et al. State of the science: cannabis and cannabinoids in palliative medicine-the potential. BMJ Support Palliat Care. 2021;11(3):299–302. https://doi.org/10.1136/bmjspcare-2021-002888.
- Nelson KM, Bisson J, Singh G, et al. The essential medicinal chemistry of cannabidiol (CBD). J Med Chem. 2020;63(21):12137–55. https://doi.org/10.1021/acs.jmedchem.0c00724.
- Zlebnik NE, Cheer JF. Beyond the CB1 receptor: is cannabidiol the answer for disorders of motivation? Ann Rev Neurosci. 2016;39:1–17. https://doi.org/10.1146/annurev-neuro-070815-014038.
- Vučković S, Srebro D, Vujović KS, et al. Cannabinoids and pain: new insights from old molecules. Front Pharmacol. 2018;9:1259. https://doi.org/10.3389/fphar.2018.01259.
- Kisková T, Mungenast F, Suváková M, et al. Future aspects for cannabinoids in breast cancer therapy. Int J Mol Sci. 2019;20:1673. https://doi.org/10.3390/ijms20071673.
- Solinas M, Massi P, Cantelmo AR, et al. Cannabidiol inhibits angiogenesis by multiple mechanisms. Br J Pharmacol. 2012 Nov;167(6):1218–31. https://doi.org/10.1111/j.1476-5381.2012.02050.x.
- Torres S, Lorente M, Rodríguez-Fornés F, et al. A combined preclinical therapy of cannabinoids and temozolomide against glioma. Mol Cancer Ther. 2011;10:90–103. https://doi.org/10.1158/1535-7163.MCT-10-0688.
- Scott KA, Dalgleish AG, Liu WM. The combination of cannabidiol and Δ9-tetrahydrocannabinol enhances the anticancer effects of radiation in an orthotopic murine glioma model. Mol Cancer Ther. 2014;13:2955–67. https://doi.org/10.1158/1535-7163.MCT-14-0402.
- López-Valero I, Torres S, Salazar-Roa M, et al. Optimization of a preclinical therapy of cannabinoids in combination with temozolomide against glioma. Biochem Pharmacol. 2018;157:275–84.
- Massi P, Vaccani A, Ceruti S, et al. Antitumor effects of cannabidiol, a non-psychotropic cannabinoid, on human glioma cell lines. J Pharmacol Exp Ther. 2003;10:255–67.
- Massi P, Valenti M, Vaccani A, et al. 5-Lipoxygenase and anandamide hydrolase (FAAH) mediate the antitumor activity of cannabidiol, a non-psychoactive cannabinoid. J Neurochem. 2008;104:1091–100. https://doi.org/10.1111/j.1471-4159.2007.05073.x.
- Solinas M, Massi P, Cinquina V, et al. Cannabidiol, a non-psychoactive cannabinoid compound, inhibits proliferation and invasion in U87-MG and T98G glioma cells through a multitarget effect. PLoS One. 2013;8:e76918. https://doi.org/10.1371/journal.pone.0076918.
- De La Ossa DHP, Lorente M, Gil-Alegre ME, et al. Local delivery of cannabinoid-loaded microparticles inhibits tumor growth in a murine xenograft model of glioblastoma multiforme. PLoS One. 2013;8:e54795. https://doi.org/10.1371/journal.pone.0054795.
- López-Valero I, Saiz-Ladera C, Torres S, et al. Targeting glioma initiating cells with a combined therapy of cannabinoids and temozolomide. Biochem Pharmacol. 2018;157:266–74. https://doi.org/10.1016/j.bcp.2018.09.007.
- Kis B, Ifrim FC, Buda V, et al. Cannabidiol – from plant to human body: a promising bioactive molecule with multi-target effects in cancer. Int J Mol Sci. 2019;20(23):5905. https://doi.org/10.3390/ijms20235905.
- Mangal N, Erridge S, Habib N, et al. Cannabinoids in the landscape of cancer. J Cancer Res Clin Oncol. 2021 Sep;147(9):2507–534. https://doi.org/10.1007/s00432-021-03710-7.
- Likar R, Koestenberger M, Stultschnig M, Nahler G. Concomitant treatment of malignant brain tumours with CBD – a case series and review of the literature. Anticancer Res. 2019;39(10):5797–801. https://doi.org/10.21873/anticanres.13783.
- Larsen C, Shahinas J. Dosage, Efficacy and safety of cannabidiol administration in adults: a systematic review of human trials. J Clin Med Res. 2020;12(3):129–41. https://doi.org/10.14740/jocmr4090.
- Britch SC, Babalonis S, Walsh SL. Cannabidiol: pharmacology and therapeutic targets. Psychopharmacology (Berl). 2021;238(1):9–28. https://doi.org/10.1007/s00213-020-05712-8.
- Taylor L, Gidal B, Blakey G, et al. A Phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018;32(11):1053–67. https://doi.org/10.1007/s40263-018-0578-5. Erratum in: CNS Drugs. 2019 Apr;33(4):397.
- Wheless JW, Dlugos D, Miller I, et al. pharmacokinetics and tolerability of multiple doses of pharmaceutical-grade synthetic cannabidiol in pediatric patients with treatment-resistant epilepsy. CNS Drugs. 2019;33(6):593–604. https://doi.org/10.1007/s40263-019-00624-4.
- Millar SA, Maguire RF, Yates AS, O'Sullivan SE. Towards better delivery of cannabidiol (CBD). Pharmaceuticals (Basel). 2020;13(9):219. https://doi.org/10.3390/ph13090219.
- Marinotti O, Sarill M. Differentiating full-spectrum hemp extracts from CBD isolates: implications for policy, safety, and science. J Diet Suppl. 2020;17(5):517–26. https://doi.org/10.1080/19390211.2020.1776806.
- Kaufmann R, Aqua K, Lombardo J, Lee M. Observed impact of long-term consumption of oral cannabidiol on liver function in healthy adults. Cannabis Cannabinoid Res. 2023;8:148–54. https://doi.org/10.1089/can.2021.0114.
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163(7):1344–64. https://doi.org/10.1111/j.1476-5381.2011.01238.x.
- Sommano SR, Chittasupho C, Ruksiriwanich W, Jantrawut P. The cannabis terpenes. Molecules (Basel, Switzerland). 2020;25(24):5792. https://doi.org/10.3390/molecules25245792.
- Seltzer ES, Watters AK, MacKenzie D Jr, et al. Cannabidiol (CBD) as a promising anti-cancer drug. Cancers (Basel). 2020;12(11):3203. https://doi.org/10.3390/cancers12113203.
- Cyr C, Arboleda MF, Aggarwal SK, et al. Cannabis in palliative care: current challenges and practical recommendations. Ann Palliat Med. 2018;7(4):463–77. https://doi.org/10.21037/apm.2018.06.04. Erratum in: Ann Palliat Med. 2019 Apr;8(2):215–7.
- Massi P, Solinas M, Cinquina V, Parolaro D. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75(2):303–12. https://doi.org/10.1111/j.1365-2125.2012.04298.x.
- Desprez PY, Murase R, Limbad C, et al. Cannabidiol treatment results in a common gene expression response across aggressive cancer cells from various origins. Cannabis Cannabinoid Res. 2021;6(2):148–55. https://doi.org/10.1089/can.2019.0081.
- Solinas M, Massi P, Cantelmo A, et al. (2012). Cannabidiol inhibits angiogenesis by multiple mechanisms. Br J Pharmacol. 2012;167(6):1218–31. https://doi.org/10.1111/j.1476-5381.2012.02050.x.
- Massi P; Valenti M; Vaccani A, et al. 5-Lipoxygenase and anandamide hydrolase (FAAH) mediate the antitumor activity of cannabidiol, a non-psychoactive cannabinoid. J. Neurochem. 2008;104:1091–100. https://doi.org/10.1111/j.1471-4159.2007.05073.x.
- Zhornitsky S, Potvin S. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals (Basel). 2012;5(5):529–52. https://doi.org/10.3390/ph5050529.
- Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30(8):1037–43. https://doi.org/10.1007/s11064-005-6978-1.
- Pertwee RG, Ross RA, Craib SJ, Thomas A. (−)-Cannabidiol antagonizes cannabinoid receptor agonists and noradrenaline in the mouse vas deferens. Eur J Pharmacol. 2002;456:99–106. https://doi.org/10.1016/s0014-2999(02)02624-9.
- Seeman P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry. 2016;6(10):e920. https://doi.org/10.1038/tp.2016.195.
- Pretzsch CM, Freyberg J, Voinescu B, et al. Effects of cannabidiol on brain excitation and inhibition systems; a randomised placebo-controlled single dose trial during magnetic resonance spectroscopy in adults with and without autism spectrum disorder. Neuropsychopharmacol. 2019;44(8):1398–405. https://doi.org/10.1038/s41386-019-0333-8.
- Fišar Z, Singh N, Hroudová J. Cannabinoid-induced changes in respiration of brain mitochondria. Toxicol Lett. 2014;231(1):62–71. https://doi.org/10.1016/j.toxlet.2014.09.002.
- Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutr Metab. 2010;7(1):7. https://doi.org/10.1186/1743-7075-7-7.
- Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013;8(21):2003–14. https://doi.org/10.3969/j.issn.1673-5374.2013.21.009.
- Martínez-Reyes I, Chandel NS. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun. 2020;11(1):102. https://doi.org/10.1038/s41467-019-13668-3.
- Porporato PE, Filigheddu N, Bravo-San Pedro JM, et al. Mitochondrial metabolism and cancer. Cell Res. 2018;28(3):265–80. https://doi.org/10.1038/cr.2017.55.
- Lu X, Zhang J, Liu H, et al. Cannabidiol attenuates pulmonary arterial hypertension by improving vascular smooth muscle cells mitochondrial function. Theranostics. 2021;11(11):5267–78. https://doi.org/10.7150/thno.55571.
- Sun S, Hu F, Wu J, Zhang S. Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol. 2017;11:577–85. https://doi.org/10.1016/j.redox.2016.12.029.
- Rimmerman N, Ben-Hail D, Porat Z, et al. Direct modulation of the outer mitochondrial membrane channel, voltage-dependent anion channel 1 (VDAC1) by cannabidiol: a novel mechanism for cannabinoid-induced cell death. Cell Death Dis. 2013;4(12):e949. https://doi.org/10.1038/cddis.2013.471.
- Murase R, Sumida T, Kawamura R, et al. Suppression of invasion and metastasis in aggressive salivary cancer cells through targeted inhibition of ID1 gene expression. Cancer Lett. 206;377(1):11–6. https://doi.org/10.1016/j.canlet.2016.04.021.
- Tang J, Gordon G, Nickoloff B et al. The helix–loop–helix protein Id-1 delays onset of replicative senescence in human endothelial cells. Lab Invest. 2002;82:1073–9. https://doi.org/10.1097/01.LAB.0000022223.65962.3A.
- Han S, Gou C, Hong L, et al. Expression and significances of Id1 helix-loop-helix protein overexpression in gastric cancer. Cancer Lett. 2004;216(1):63–71. https://doi.org/10.1016/j.canlet.2004.07.035.
- Ling MT, Wang X, Zhang X, Wong YC. The multiple roles of Id-1 in cancer progression. Differentiation. 2006;74(9–10):481–7. https://doi.org/10.1111/j.1432-0436.2006.00083.x.
- McAllister SD, Murase R, Christian RT, et al. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res Treat. 2010;129(1):37–47. https://doi.org/10.1007/s10549-010-1177-4.
- Elbaz M, Nasser MW, Ravi J, et al. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: novel anti-tumor mechanisms of cannabidiol in breast cancer. Mol Oncol. 2015;9(4):906–19. https://doi.org/10.1016/j.molonc.2014.12.010.
- Nicholson R, Gee, JM, Harper M. EGFR and cancer prognosis. Eur J Cancer. 2001;37:9–15. https://doi.org/10.1016/s0959-8049(01)00231-3.
- Milian L, Mata M, Alcacer J, et al. Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS One. 2020;15(2):e0228909. https://doi.org/10.1371/journal.pone.0228909.
- Fiandalo MV, Kyprianou N. Caspase control: protagonists of cancer cell apoptosis. Exp Oncol. 2012;34(3):165–75.
- Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21(3):485–95. https://doi.org/10.1093/carcin/21.3.485.
- Park YJ, Na HH, Kwon IS, et al. Cannabidiol regulates PPARγ-dependent vesicle formation as well as cell death in A549 human lung cancer cells. Pharmaceuticals (Basel). 2022;15(7):836. https://doi.org/10.3390/ph15070836.
- McKallip RJ. Cannabidiol-induced apoptosis in human leukemia cells: a novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol Pharmacol. 2006;70(3):897–908. https://doi.org/10.1124/mol.106.023937.
- Jeong S, Jo MJ., Yun HK. et al. Cannabidiol promotes apoptosis via regulation of XIAP/Smac in gastric cancer. Cell Death Dis. 2019;10:846. https://doi.org/10.1038/s41419-019-2001-7.
- Shrivastava A, Kuzontkoski PM, Groopman JE, Prasad A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol Cancer Ther. 2011;10(7):1161–72. https://doi.org/10.1158/1535-7163.MCT-10-1100.
- Marleau AM., Chen, CS., Joyce JA. et al. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012;10:134. https://doi.org/10.1186/1479-5876-10-134.
- Wang J, Zheng Y, Zhao M. Exosome-based cancer therapy: implication for targeting cancer stem cells. Front Pharmacol. 2017;7:533. https://doi.org/10.3389/fphar.2016.00533.
- Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem. 2016;74:103–41. https://doi.org/10.1016/bs.acc.2015.12.005.
- Kosgodage US, Mould R, Henley AB, et al. Cannabidiol (CBD) Is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front Pharmacol. 2018;9:889. https://doi.org/10.3389/fphar.2018.00889.
- Kosgodage US, Uysal-Onganer P, MacLatchy A, et al. Cannabidiol affects extracellular vesicle release, miR21 and miR126, and reduces prohibitin protein in glioblastoma multiforme cells. Transl Oncol. 2019;12(3):513–22. https://doi.org/10.1016/j.tranon.2018.12.004.
- Perkins D, Butler J, Ong K, et al. A Phase 1, randomised, placebo-controlled, dose escalation study to investigate the safety, tolerability and pharmacokinetics of cannabidiol in fed healthy volunteers. Eur J Drug Metab Pharmacokinet. 2020;45(5):575–86. https://doi.org/10.1007/s13318-020-00624-6.
- Schoeman R, Beukes N, Frost C. Cannabinoid combination induces cytoplasmic vacuolation in MCF-7 breast cancer cells. Molecules. 2020;25(20):4682. https://doi.org/10.3390/molecules25204682.
- Khodadadi H, Salles ÉL, Alptekin A, et al. Inhalant cannabidiol inhibits glioblastoma progression through regulation of tumor microenvironment. Cannabis Cannabinoid Res. 2021. https://doi.org/10.1089/can.2021.0098. Epub ahead of print.
- Romano B, Borrelli F, Pagano E, et al. Inhibition of colon carcinogenesis by a standardized Cannabis sativa extract with high content of cannabidiol. Phytomedicine. 2014;21(5):631–9. https://doi.org/10.1016/j.phymed.2013.11.006.
- Mechoulam R. Chemistry of cannabis. Handb Exp Pharmacol. 1981;55:119–234.
- van Drooge DJ, Hinrichs WL, Wegman KA, et al. Solid dispersions based on inulin for the stabilisation and formulation of delta 9-tetrahydrocannabinol. Eur J Pharm Sci. 2004;21(4):511–8. https://doi.org/10.1016/j.ejps.2003.11.014.
- Fraguas-Sánchez AI, Fernández-Carballido A, Simancas-Herbada R, et al. CBD loaded microparticles as a potential formulation to improve paclitaxel and doxorubicin-based chemotherapy in breast cancer. Int J Pharm. 2020;574:118916. https://doi.org/10.1016/j.ijpharm.2019.118916.
- Fraguas-Sánchez AI, Fernández-Carballido A, Delie F, et al. Enhancing ovarian cancer conventional chemotherapy through the combination with cannabidiol loaded microparticles. Eur J Pharm Biopharm. 2020;154:246–58. https://doi.org/10.1016/j.ejpb.2020.07.008.
- Fraguas-Sánchez AI, Torres-Suárez AI, Cohen M, et al. PLGA nanoparticles for the intraperitoneal administration of CBD in the treatment of ovarian cancer: in vitro and in ovo assessment. Pharmaceutics. 2020;12(5):439. https://doi.org/10.3390/pharmaceutics12050439.
- Nabissi M, Morelli MB, Santoni M, Santoni G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis. 2013;34:48–57. https://doi.org/10.1093/carcin/bgs328.
- Deng L, Ng L, Ozawa T, Stella N. Quantitative analyses of synergistic responses between cannabidiol and DNA-damaging agents on the proliferation and viability of glioblastoma and neural progenitor cells in culture. J Pharmacol Exp Ther. 2017;360:215–24. https://doi.org/10.1124/jpet.116.236968.
- 100. Good P, Haywood A, Gogna G, et al. Oral medicinal cannabinoids to relieve symptom burden in the palliative care of patients with advanced cancer: a double-blind, placebo controlled, randomised clinical trial of efficacy and safety of cannabidiol (CBD). BMC Palliat Care. 2019;18(1):110. https://doi.org/10.1186/s12904-019-0494-6.