Endometriosis is one of the most destructive benign diseases of women. It is established as developing and being present in upward of 70% of adolescents who do not experience relief of menstrual pain with use of oral contraceptives and anti-inflammatory drugs. It occurs in 8%–10% of women in the United States and is most prevalent in developed countries. Symptoms of endometriosis include disabling pain, hemorrhagic uterine bleeding, and infertility. Women with disease can expect a 12% hysterectomy rate. While present medical therapy may offer relief of many symptoms, there have been no major new directions in pharmacologic therapy since leuprolide acetate was made available in 1977. Danazol remains the only alternative to GnRH agonists with proven efficacy and reasonable side effects, according to Cochrane Reviews, yet, it is underused, and GnRH agonists are favored even when Danazol in combination seems more effective. A previously published case report on use of the combination of nandrolone and stanozolol to treat a young woman scheduled for hemicolectomy is discussed as an alternative to surgery along with the limits of standard therapy. This review will focus on recent research and theories seeking to establish causation for disease and offer treatment recommendations.
Endometriosis is one of the most destructive benign diseases of women. It is established as developing and being present in upwards of 70% of adolescents who fail to have relief of menstrual pain with use of oral contraceptives and anti-inflammatory drugs.1 It occurs in 8%−10% of women in the United States2 and is most prevalent in developed countries. Symptoms of endometriosis include disabling pain, hemorrhagic uterine bleeding, and infertility. Women with disease can expect a 12% hysterectomy rate.3
While present medical therapy may offer relief of many symptoms, there have been no major new directions in pharmacologic therapy since leuprolide acetate was made available in 1977.4 Although leuprolide acetate, a gonadotropin-releasing hormone (GnRH) agonist, suppresses biologically produced estrogen levels below measurable levels, the side effects of estrogen deprivation limit this drug’s long-term effectiveness. Previous medications used for endometriosis include oral contraceptives,5 cyclic estradiol with medroxyprogesterone acetate (MPA) or tribolone,6 MPA monthly intramuscular injections,7 megesterol,8 the levonorgestrel intrauterine device (and all progestins),9 and testosterone.10 These are less effective than both GnRH agonists and danazol.11 Danazol remains the only alternative to GnRH agonists with proven efficacy and reasonable side effects, according to Cochrane Reviews9,11; yet, it is underused, and GnRH agonists are favored.
Recent attempts to use add-back estrogen, aromatase inhibitors, anti–tumor necrosis factor-α, and dienogest to minimize signs and symptoms of GnRH agonist use offer no improvement in therapy and add extra cost.12 Failure of these therapies13–15 results in surgery, often hysterectomy or oophorectomy, and in estrogen deprivation medical therapy. Furthermore, the incidence of comorbid diseases16–19 remains significant; long-term sequelae include ovarian cancer, endometrial cancer, and cerebral infarction.20 A case report on use of the combination of nandrolone and stanozolol to treat a young woman scheduled for hemicolectomy is discussed as an alternative to surgery along with the limits of standard therapy.
R. L. Barbieri of Boston’s Brigham and Women’s Hospital states, “The ideal drug regimen for the treatment of endometriosis remains to be developed.”21 Attempts were made 20 years ago to use add-back estrogen and a synthetic progestin to reduce bone loss,13 but this therapy often resulted in return of the signs and symptoms of the original disease. Women still may not experience relief of endometriosis symptoms of pain, even after adding back of the litany of synthetic drugs,13 notwithstanding their additional set of risks and complications. Ignored since 1990 is the combination of gonadotropin-releasing hormone (GnRH) and danazol, which was found to be superior to use of each therapy alone in inducing the regression of fluid in experimental endometriosis.14 Long-term sequelae of estrogen deprivation may include increases in mortality,15 heart disease,16 osteoporosis,17 and loss of sexual function,18 as well as decreased quality of life.19 A need for a new direction in understanding and treatment of this disease exists. The only steps taken to date have been (1) medical therapy focused on suppression of pituitary suppression of ovarian estrogen production with medications such as dienogest and (2) medical treatment of the signs and symptoms of disease.One focus of this review is the recent research seeking to establish causation for diseases. The term for it is epigenetics: epi- for epidemiologic and genetics for genetic factors. Both animal and human studies offer supporting, albeit not absolute, proof that exposure to environmental estrogenic compounds can be linked to the development of endometriosis.22 The cells of patients with endometriosis are found to have postconception genetic changes in DNA and messenger RNA (mRNA) alterations.23 It is suggested that sex hormone-binding globulin (SHBG) may transport some contaminant xenoestrogens into the plasma and modulate their bioavailability to cell tissues.24 What is not commonly considered is that the estrogenic potential of the xenoestrogens and their metabolites can be just as potent as 17β-estradiol.25 The author’s hypothesis is that these environmental and/or synthetic xenoestrogens can potentially be blocked from penetrating the androgen receptor (AR), thereby preventing their intracellular availability. This is accomplished by using pharmaceutical compounds that have significantly greater AR affinity26 than the xenoestrogens, thus keeping the xenoestrogens from entering the cell and the nucleus and propagating mRNA and DNA. Accepting the fact that endometriosis is inflammatory and catabolic (i.e. destructive), the previous medical literature of treatment identified only two courses of therapy, both of which were both effective and anabolic: testosterone and danazol. These anabolic therapies are shown to positively influence the bioavailability of testosterone, which is measured as the first component of the Free Androgen Index (FAI), proposed by Burke and Anderson in 1972.27 The FAI increases with increasing concentration of testosterone and with decreases in the second component, SHBG. A higher FAI occurs with danazol28,29 therapy and in natural conditions of high testosterone production (polycystic ovarian disease).30,31 All forms of biological and synthetic estrogens decrease the FAI by lowering serum testosterone and increasing SHBG.25,32,33The author’s previously published case report34 explained that the androgen nandrolone, by displacing testosterone and stanozolol and limiting SHBG production, offers a potentially unrecognized modality to dramatically increase the FAI.35 On the basis of follow-up in this case, this mixture of anabolic compounds remains an effective long-term treatment for even the most extensive endometriosis disease. Furthermore, this novel medication combination acts similarly to danazol (in raising testosterone and decreasing SHBG) without the severe, dose-related hirsutism side effects of high-dose danazol. The novel medical mixture results in upward of 50 times increases in the FAI over that of danazol. On the basis of this case report, this anabolic combination can potentially avoid both the side effects of leuprolide acetate and high-dose danazol and could be a compassionate alternative to radical surgery in patients with advanced and resistant endometriosis.
Endometriosis is defined as the presence of endometrial gland and stroma outside the uterine cavity.36 Endometriosis is a disease that affects 176–200 million women worldwide.37,38 Twelve percent of women with endometriosis will undergo surgery; upward of 100,000 hysterectomies are performed annually in the United States for this disease. Moreover, half of all the hysterectomies are found to have related signs and symptoms of endometriosis: endometriosis proper, 16.2%; symptom complex of uterine bleeding, 25.2%; and pain, 11.7%.39 Surgery cannot prevent the 10% recurrence rate, the 20%–40% left with residual pain, or the 3.7% reoperation rate after castration (hysterectomy, oophorectomy).40 A high recurrence rate of 62% is reported in advanced stages of endometriosis in which the ovaries are conserved.41 Ovarian conservation carries a six-fold risk of recurrent pain and an eight-fold risk of reoperation.41 Most will experience premature menopause without the option of estrogen replacement therapy (ERT), as ERT may often stimulate endometriosis recurrence.41
Since 1921, eight theories have been presented as the singular causative component of endometriosis. While not one of those theories is all-inclusive, the ninth – environmental toxins and/or xenoestrogens – may explain the majority of these observations brought forward from previous theories.
Eight hypotheses of the cause of endometriosis have been proposed. They are outlined in the subsections that follow.
Sampson Theory of Retrograde MenstruationAt the time of menstruation, there can be backflow of menstrual debris through the fallopian tubes.42 It has been postulated that hereditary factors, toxins, or a compromised immune system is to blame for this process. Redwine stated that the implants seen in women with endometriosis are dissimilar from those of normal retrograde menstruation.43 The amount of retrograde menstruation noted by Barbieri44 is dependent on the diameter of the cervical opening. Woodbury45 in the 1940s, Csapo46 in the 1960s, and Lichten (unpublished data) in the 1990s showed that the obstruction was physiological and not mechanical. The temporary contraction ring at the cervical junction is associated with high-amplitude intrauterine pressure waves that could be relieved by a paracervical block (E. M. Lichten, MD, unpublished data, 1990). Endometriosis is associated with subclinical inflammatory biomarkers within the peritoneal cavity. It may be speculated that proinflammatory stimuli strong enough to cause an increase in acute inflammatory phase protein peritoneal fluid concentrations47 that stimulates these parasympathetic paracervical nerves, leading to the junctional contraction ring. Furthermore, despite a normal laparoscopic appearance, microscopic endometriosis, endosalpingiosis, and inflammatory changes were found in uterosacral ligaments in 17 (63%) women with chronic pelvic pain.48 This observation correlates with the relief of pain after surgically blocking the uterosacral or presacral nerves, thereby relieving the aforementioned muscle spasms previously described as in the aftermath of the contraction ring. Pain relief may last for one menstrual cycle with a local anesthetic paracervical or epidural block, or for months after surgical interruption of the nerve pathways by laparoscopic uterine nerve ablation49 or presacral neurectomy.50
The link to biochemical changes and toxic chemicals is traced to Moghissi, who first identified that women with endometriosis and/or dysmenorrhea produced an excess of prostaglandin51 as measured in menstrual debris. The amount of prostaglandin relates directly to severity of pain. The rationale is that the increased intrauterine pressure resulting from cervical obstruction produces more prostaglandin release from the menstrual debris retained within the uterus. Excess prostaglandins are found in the peritoneal fluid as well.52 This retrograde menstruation theory, although clinically accurate, cannot explain why the serum concentration of dioxin substances53 is greater in women with peritoneal endometriosis and greatest in those with deep infiltrating endometriosis. Concluding that higher exposure to dioxin xenoestrogen toxins are the original insult that produces endometriosis, the author proposes the following pathway to explain these described observations. The endometriosis first induces the presence of abnormal biomarkers,54 including prostaglandins released by peritoneal endometriosis. Prostaglandins then irritate the uterosacral nerves, which causes the physiological obstruction at the cervix, followed by the retained menstrual debris; retrograde menstruation; and high-amplitude contraction, spasm, and pain.
Imperforate hymen55 and other anatomical obstructions of menstrual egress can be associated with disease. The occurrence of these is rare.
An increased incidence of endometriosis has been noted within families in epidemiologic studies.56 Changes in chromosomes 1, 2, 6, 7, 9, 10, and 12 have been associated with endometriosis.57 Recent studies have identified genetic links between endometriosis and specific ovarian cancers.58 The increased incidence of endometriosis being noted in areas of high xenoestrogen exposure59 raises the question whether the genetic defect developed first in the mother because of her prior exposure, or was caused by the de novo exposure of the offspring to xenoestrogen in utero. In either case, the link of endometriosis and xenoestrogens such as dioxin,60 at least in select cases, has been established.
The mullerianosis61 theory of endometriosis proposes that cells within the Müllerian tract act as stem cells. Although supported by fetal autopsy, ectopic endometriosis as it appears in the lung cannot conform to this theory.
The coelomic metaplasia62 theory suggests that these common ancestral cells of the endometrial and peritoneal cells transform into endometrial cells triggered by inflammation. This theory does not explain where the inflammation originates, the role of xenoestrogens, or the observations addressed by other theories.
Thirty-seven percent of the microvascular endometrium of ectopic endometrial tissue originates from endothelial progenitor cells.63 This theory does not explain where the inflammation originates, the role of xenoestrogens, or the other observations addressed by other theories.
Neural growth is noted in sensory C, sensory Aδ, sympathetic, and parasympathetic nerve fibers in the functional layer of the endometrium of most women affected by endometriosis, but it is not demonstrated in most women who do not have endometriosis.64
Progesterone resistance has been noted in endometrial fibroblasts derived from mesenchymal progenitors in cases of endometriosis.65 There are growth factor66 abnormalities in the endometriosis tissue as well. Numerous other genetically induced molecular abnormalities have also been noted.67 The end result is that the normal suppression of endometrial tissue by natural progesterone does not occur. This concurs with Redwine’s43 finding that endometriosis tissue differs from retrograde menstruation.There is ample evidence connecting endometriosis to xenoestrogens. Jurewicz went further when he stated that “in all the reviewed studies, exposure to phthalates adversely affected the level of reproductive hormones (luteinizing hormone, free testosterone, sex hormone-binding globulin), anogenital distance and thyroid function.”68 The urinary levels of phthalates were significantly higher in the pubertal gynecomastia group and in serum of girls with premature thelarche and precocious puberty. Epidemiological studies, in spite of their limitations, suggest that xenoestrogens, including phthalates, dioxin, and organochlorides, may affect reproductive outcome and children’s health.69–71
The author has chosen to use the older term, xenoestrogens, to focus on the estrogenic characteristics of these man-made toxins, specifically, how they penetrate the cell at the estrogen receptor (i.e. androgen receptor). The author avoids broader terminology that includes endocrine-disrupting chemicals (EDCs) because EDCs influence thyroid and adrenal hormone receptors as well as testosterone and estrogen production and receptors.
The new epigenetic research links human exposure to xenoestrogens to endometriosis, reproductive outcome,70 children’s health,69,70 and other female conditions, including uterine fibroids.67,70 Sofo22 clearly put forward the theoretic steps between dioxin exposure and endometriosis, while Cai60 explained the immunological changes that prevent apoptosis in endometriosis:
- The correlation between dioxin and endometriosis22 is an epigenetic route to unravel the pathogenesis of the disease. Environmental toxicants can act as endocrine disrupters on the female reproductive system. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is resistant to degradation and due to its lipophilic nature, accumulates in the fat tissue and in the food chain. Human and animal exposure to TCDD affects levels of the steroid receptors and steroid-responsive gene expression and has an impact on metabolism and serum transport of steroids. Gene expression is commonly altered in endometriosis and in the eutopic endometrium of women with the disease. Aberrantly expressed genes include those associated with the regulation of transcription, proliferation, sex steroid metabolism, apoptosis, cell cycle, the immune response and cell adhesion.22
- Alterations within the immune system of endometriosis patients include that endometrial cells from women with this disease not only have the capacity to escape immune-surveillance, but also use inflammatory mechanisms to promote their growth within the peritoneal cavity.65
- There is evidence that exposure to an environmental endocrine disruptor, such as 2,3,7,8-tetracholorodibenzo-p-dioxin, can mediate the development of an endometrial phenotype that exhibits both reduced progesterone responsiveness and hypersensitivity to pro-inflammatory stimuli mimicking the endometriosis phenotype.60
SHBG72 circulates freely in the serum. Nandrolone binds freely to SHBG. Anderson27 stated, “In men, [the AR binding site] is nearly saturated since the molar concentration of SHBG binding sites in adult male plasma is only marginally greater than the molar concentration of testosterone. In female plasma, the SHBG concentration is two-fold higher and the testosterone concentration tenfold lower than in men and therefore most of the binding sites are unoccupied.”27 That is why there is a 20-fold difference in the FAI between men and women of reproductive age. The serum concentration of SHBG increases in states of estrogen excess: pregnancy,73 synthetic estrogens (oral contraception),74 etonogestrel/ethinyl estradiol vaginal ring, menopausal conjugated estrogens,75 and exposure to certain EDCs.76 Endometriosis is also a state related to SHBG excess.28 Elevated levels of SHBG competitively inhibit the availability of nandrolone to bind to the AR.
It has been the author’s experience that higher serum levels of SHBG are noted in cases of endometriosis than in control subjects, with the highest levels being associated with the most extensive disease. Panidis28 reported these same findings in 1993. At the cellular level, increased ovarian SHBG receptors are noted with endometriosis.77 Taking these SHBG observations in combination with those of Barbieri,78 who stated, “Differences in the molecular, endocrine, and clinical pharmacologic properties of these agents (GnRH and danazol) may provide clues to their varying effects in the management of women with endometriosis,” the author predicts that an effective modality to treat endometriosis will be one that lowers SHBG and uses anabolic androgens to raise the FAI. Before the publication of the previously published case report,34 the only modality that accomplished both was danazol (see Table 1). While danazol temporarily increases the FAI (testosterone/SHBG ratio) by two- to three-fold, it soon reaches equilibrium at 25%–50% above baseline. The case report documents a greater than 50-fold increase in the FAI with weekly intramuscular injections of 40 mg of nandrolone and 15 mg of stanozolol. Side effects are minimal, as the FAI remains unchanged 8 years after initial therapy.
Table 1
Effect of medical treatments for endometriosis on biological parameters.
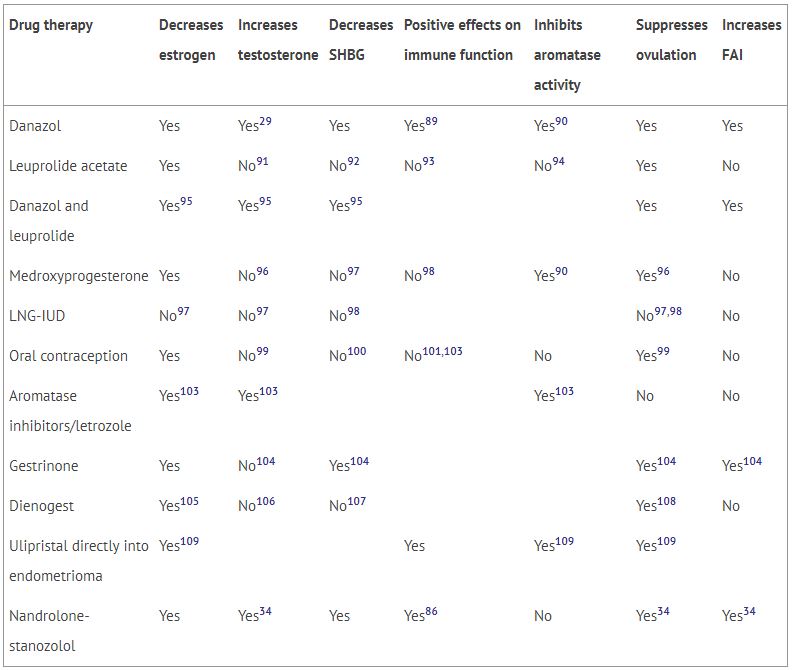
| Drug therapy | Decreases estrogen | Increases testosterone | Decreases SHBG | Positive effects on immune function | Inhibits aromatase activity | Suppresses ovulation | Increases FAI |
|---|---|---|---|---|---|---|---|
| Danazol | Yes | Yes29 | Yes | Yes89 | Yes90 | Yes | Yes |
| Leuprolide acetate | Yes | No91 | No92 | No93 | No94 | Yes | No |
| Danazol and leuprolide | Yes95 | Yes95 | Yes95 | Yes | Yes | ||
| Medroxyprogesterone | Yes | No96 | No97 | No98 | Yes90 | Yes96 | No |
| LNG-IUD | No97 | No97 | No98 | No97,98 | No | ||
| Oral contraception | Yes | No99 | No100 | No101,103 | No | Yes99 | No |
| Aromatase inhibitors/letrozole | Yes103 | Yes103 | Yes103 | No | No | ||
| Gestrinone | Yes | No104 | Yes104 | Yes104 | Yes104 | ||
| Dienogest | Yes105 | No106 | No107 | Yes108 | No | ||
| Ulipristal directly into endometrioma | Yes109 | Yes | Yes109 | Yes109 | |||
| Nandrolone-stanozolol | Yes | Yes34 | Yes | Yes86 | No | Yes34 | Yes34 |
Nandrolone35 is superior to danazol because it has a three-fold greater affinity for the AR than testosterone and 30 times greater affinity for the AR than any estrogenic compound. Danazol’s binding to the AR is much less potent. Nandrolone is not metabolized as testosterone is to estradiol, nor to dihydrotestosterone.
Stanozolol79 is used to block the liver production of SHBG. It is a first derivative of naturally occurring dihydrotestosterone. Stanozolol lowers SHBG by 50% in 1 week79 and by 80%–90% at 8 weeks.79 By lowering SHBG, which competes with the AR binding, nandrolone’s availability for AR binding is maximized. While nandrolone reduces natural estradiol and testosterone production by the feedback loop inhibition of follicle-stimulating hormone and luteinizing hormone,80 it also displaces testosterone from the AR.35 Measurements of the effect of therapy are seen in the increase in the FAI as the serum testosterone increases and SHBG decreases. There are no direct standard laboratory measurements of nandrolone. This combination therapy of U.S. Food and Drug Administration–approved nandrolone and stanozolol medications appears to be safe and effective even after the failure of surgery in advanced cases of endometriosis. In the range of effective dosages, these medications are showing none of the more severe side effects of standard medical therapy and are expected to maintain muscle and bone integrity81 and lean mass82 and to minimize menopausal symptoms, including LH-induced hot flushes.80
It is the stated goal of medical therapy to reduce estrogen levels in endometriosis. Dickey noted in 1984 that “complete or almost complete remission [in endometriosis] occurred when radioimmunoassays of estradiol (E2) levels were less than 15 pg/ml in extensive, less than 22 pg/ml in severe, and less than 41 pg/ml in moderate endometriosis.”83 However, Dickey’s report84 does not correlate with the 176% increase incidence of hysterectomy and/or oophorectomy reported for endometriosis, documented by the CDC from 1965 to 1984,85 and continuing subsequently. In personal discussion in October 2014, Dickey noted that he had used dexamethasone to reduce adrenal estrone in severe cases of endometriosis. Whether xenoestrogen exposure since 1974 could explain the increase in endometriosis occurrence and the increase in related hysterectomy and/or oophorectomy that follows is subject to conjecture.
Exposure to xenoestrogens can explain causation of endometriosis. The theory includes the documented increased exposure today to environmental toxins, epigenetic chromosomal breaks, decreased immunology with increased inflammatory markers, and changes in serum SHBG and tissue receptor binding in endometriosis lesions. Medical treatment needs to consider all the previous biological parameters to accomplish the following:
- Lower serum estrogen levels as proposed by Dickey83
- Block all in vivo and in vitro estrogens from entry at the AR with nandrolone as proposed by the author
- Direct suppression of SHBG with danazol28 or, preferably, stanozolol79
- Reduce inflammatory markers86
Danazol alone or in combination with GnRH analogues is many times more expensive and has a multitude of side effects not witnessed with the novel combination of nandrolone and stanozolol. Danazol remains more cost-effective and better-tolerated for treating milder cases and for those patients who wish to avoid weekly injections. The improvement in endometriosis with both therapies corresponds to the increase in the FAI. The previously mentioned case report shows that the effectiveness of combined nandrolone-stanozolol in cases of advanced endometriosis is more profound because it induces greater increases in FAI. Laboratory assays of FAI in serum provide a more effective test of endometriosis relief than does serum estradiol alone. A large independent board-supervised review study is needed to confirm that these findings are reproducible.
- Propst AM,
Laufer MR.
Endometriosis in adolescents: incidence, diagnosis, and treatment.
- Trabert B,
De Roos AJ,
Schwartz SM, et al.
Non-dioxin-like polychlorinated biphenyls and risk of endometriosis.
- Keshavarz H,
Hillis SD,
Kieke BA,
Marchbanks PA.
Hysterectomy surveillance – United States, 1994–1999.
- CenterWatch.
Lupron Depot (leuprolide acetate for depot suspension) [accessed July 20, 2016].
Available from: http://www.centerwatch.com/drug-information/fda-approved-drugs/drug/293/lupron-depot-leuprolide-acetate-for-depot-suspension - Muzii L,
Di Tucci C,
Achilli C, et al.
Continuous versus cyclic oral contraceptives after laparoscopic excision of ovarian endometriomas: a systematic review and metaanalysis.
- Al Kadri H,
Hassan S,
Al-Fozan HM,
Hajeer A.
Hormone therapy for endometriosis and surgical menopause.
- Crosignani PG,
Luciano A,
Ray A,
Bergqvist A.
Subcutaneous depot medroxyprogesterone acetate versus leuprolide acetate in the treatment of endometriosis-associated pain.
- Schlaff WD,
Dugoff L,
Damewood MD,
Rock JA.
Megestrol acetate for treatment of endometriosis.
- Brown J,
Kives S,
Akhtar M.
Progestagens and anti-progestagens for pain associated with endometriosis.
- Rencoret R,
Vargas L.
Persistent, obstructive, and recurrent intestinal endometriosis; regression by prolonged treatment with testosterone; report of a case.
- Brown J,
Farquhar C.
Endometriosis: an overview of Cochrane Reviews.
- Kim SH,
Chae HD,
Kim CH,
Kang BM.
Update on the treatment of endometriosis.
- Edmonds DK.
Add-back therapy in the treatment of endometriosis: the European experience.
- Sakata M,
Terakawa N,
Mizutani T, et al.
Effects of danazol, gonadotropin-releasing hormone agonist, and a combination of danazol and gonadotropin-releasing hormone agonist on experimental endometriosis.
- Gierach GL,
Pfeiffer RM,
Patel DA, et al.
Long-term overall and disease-specific mortality associated with benign gynecologic surgery performed at different ages.
- Mu F,
Rich-Edwards J,
Rimm EB, et al.
Endometriosis and risk of coronary heart disease.
- Matsuo H.
Bone loss induced by GnRHa treatment in women [in Japanese].
- Fritzer N,
Tammaa A,
Haas D, et al.
When sex is not on fire: a prospective multi-centre study evaluating the short-term effects of radical resection of endometriosis on quality of sex life and dyspareunia.
- Gao X,
Yeh YC,
Outley J, et al.
Health-related quality of life burden of women with endometriosis: a literature review.
- Nagai K,
Hayashi K,
Yasui T, et al.
Disease history and risk of comorbidity in women’s life course: a comprehensive analysis of the Japan Nurses’ Health Study baseline survey.
- Barbieri RL.
Endometriosis 1990: current treatment approaches.
- Sofo V,
Götte M,
Laganà AS, et al.
Correlation between dioxin and endometriosis: an epigenetic route to unravel the pathogenesis of the disease.
- Koike N,
Higashiura Y,
Akasaka J, et al.
Epigenetic dysregulation of endometriosis susceptibility genes.
- Déchaud H,
Ravard C,
Claustrat F, et al.
Xenoestrogen interaction with human sex hormone-binding globulin (hSHBG).
- Rubin BS.
Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects.
- Pugeat M.
Mechanism of action and peripheral metabolism of androgens in women [in French].
- Burke CW,
Anderson DC.
SHBG is an oestrogen amplifier.
- Panidis D,
Kokkinos T,
Vavilis D, et al.
SHBG serum levels in women with endometriosis before, during and after long-term danazol therapy [in German].
- Forbes KL,
Dowsett M,
Rose GL, et al.
Dosage-related effects of danazol on sex hormone binding globulin and free and total androgen levels.
- Daan NM,
Jaspers L,
Koster MP, et al.
Androgen levels in women with various forms of ovarian dysfunction: associations with cardiometabolic features.
- Bui HN,
Sluss PM,
Hayes FJ, et al.
Testosterone, free testosterone, and free androgen index in women: reference intervals, biological variation, and diagnostic value in polycystic ovary syndrome.
- Campagnoli C,
Lesca L,
Cantamessa C,
Peris C.
Long-term hormone replacement treatment in menopause: new choices, old apprehensions, recent findings.
- Wiegratz I,
Jung-Hoffmann C,
Kuhl H.
Effect of two oral contraceptives containing ethinylestradiol and gestodene or norgestimate upon androgen parameters and serum binding proteins.
- Lichten EM.
Novel medical protocol offers alternative to total abdominal hysterectomy with bilateral salpingo-oophorectomy and hemicolectomy in stage IV endometriosis.
- Saatok T,
Dahlberg E,
Gustafsson JA.
Relative binding affinity of anabolic-androgenic steroids: comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin.
- Agarwal N,
Subramanian A.
Endometriosis – morphology, clinical presentations and molecular pathology.
- Wood R,
Guidone H,
Hummelshoj L.
Myths and misconceptions in endometriosis.
http://endometriosis.org/resources/articles/myths/. Accessed July 20, 2016 . - Merrill RM.
Hysterectomy surveillance in the United States, 1997 through 2005.
- Cleveland Clinic.
Recurrent endometriosis: surgical management. Accessed July 20, 2016. - Rizk B,
Fischer AS,
Lotfy HA, et al.
Recurrence of endometriosis after hysterectomy.
- Benagiano G,
Brosens I,
Lippi D.
The history of endometriosis.
- Redwine DB.
Was Sampson wrong? - Barbieri RL,
Callery M,
Perez SE.
Directionality of menstrual flow: cervical os diameter as a determinant of retrograde menstruation.
- Woodbury RA,
Child GP.
Electro-uterography and the physiology of the human uterus as related to dysmenorrhea and metrorrhagia.
- Csapo AI.
A rationale for the treatment of dysmenorrhea.
- Polak G,
Barczyński B,
Bednarek W, et al.
Increased levels of proteins of the acute inflammatory phase in the peritoneal fluid of women with advanced stages of endometriosis.
- Nascu PC,
Vilos GA,
Ettler HC, et al.
Histopathologic findings on uterosacral ligaments in women with chronic pelvic pain and visually normal pelvis at laparoscopy.
- Lichten EM,
Bombard J.
Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation.
- Jedrzejczak P,
Sokalska A,
Spaczyński RZ, et al. Effects of presacral neurectomy on pelvic pain in women with and without endometriosis.
- Moghissi KS.
Prostaglandins in reproduction.
- De Leon FD,
Vijayakumar R,
Brown M, et al.
Peritoneal fluid volume, estrogen, progesterone, prostaglandin, and epidermal growth factor concentrations in patients with and without endometriosis.
- Heilier JF,
Nackers F,
Verougstraete V, et al.
Increased dioxin-like compounds in the serum of women with peritoneal endometriosis and deep endometriotic (adenomyotic) nodules.
- Gupta D,
Hull ML,
Fraser I, et al.
Endometrial biomarkers for the non-invasive diagnosis of endometriosis.
- Lardenoije C,
Aardenburg R,
Mertens H.
Imperforate hymen: a cause of abdominal pain in female adolescents.
- Kennedy S,
Mardon H,
Barlow D.
Familial endometriosis.
- Kobayashi H,
Imanaka S,
Nakamura H,
Tsuji A.
Understanding the role of epigenomic, genomic and genetic alternations in the development of endometriosis.
- Lu Y,
Cuellar-Partida G,
Painter JN, et al.
Shared genetics underlying epidemiological association between endometriosis and ovarian cancer.
- Benagiano G,
Brosens I.
In utero exposure and endometriosis.
- Cai LY,
Izumi S,
Suzuki T, et al.
Dioxins in ascites and serum of women with endometriosis: a pilot study.
- Troncon JK,
Zani AC,
Vieira AD, et al.
Endometriosis in a patient with Mayer-Rokitansky-Küster-Hauser syndrome.
- Suginami H.
A reappraisal of the coelomic metaphasia theory by reviewing endometriosis occurring in unusual sites and instances.
- Laschke MV,
Giebels C,
Menger MD.
Vasculogenesis: a new piece of the endometriosis puzzle.
- Miller EJ,
Fraser IS.
The importance of pelvic nerve fibers in endometriosis.
- Barragan F,
Irwin JC,
Balayan S, et al.
Human endometrial fibroblasts derived from mesenchymal progenitors inherit progesterone resistance and acquire an inflammatory phenotype in the endometrial niche in endometriosis.
- Bruner KL,
Eisenberg E,
Gorstein F,
Osteen KG.
Progesterone and transforming growth factor-β coordinately regulate suppression of endometrial matrix metalloproteinases in a model of experimental endometriosis.
- Bulun SE,
Monsivais D,
Kakinuma T, et al.
Molecular biology of endometriosis: from aromatase to genomic abnormalities.
- Jurewicz J,
Hanke W.
Exposure to phthalates: reproductive outcome and children health. A review of epidemiological studies.
- Heilier JF,
Donnez J,
Lison D.
Organochlorines and endometriosis: a mini-review.
- Gerhard I,
Runnebaum B.
The limits of hormone substitution in pollutant exposure and fertility disorders [in German].
- Misao R,
Nakanishi Y,
Fujimoto J,
Tamaya T.
Expression of sex hormone-binding globulin exon VII splicing variant messenger ribonucleic acid in human ovarian endometriosis.
- Thomas JA.
- Mean F,
Pellaton M,
Magrini G.
Study on the binding of dihydrotestosterone, testosterone and oestradiol with sex hormone binding globulin.
- Zimmerman Y,
Eijkemans MJ,
Coelingh Bennink HJ, et al.
The effect of combined oral contraception on testosterone levels in healthy women: a systemic review and meta-analysis.
- Sood R,
Faubion SS,
Kuhle CL, et al.
Prescribing menopausal hormone therapy: an evidence-based approach.
- Michalek JE,
Akhtar FZ,
Kiel JL, et al.
Serum dioxin, insulin, fasting glucose, and sex hormone-binding globulin in veterans of Operation Ranch Hand.
- Misao R,
Hori M,
Ichigo S, et al.
Levels of sex hormone-binding globulin (SHBG) and corticosteroid-binding globulin (CBG) messenger ribonucleic acid (mRNAs) in ovarian endometriosis.
- Barbieri RL.
Comparison of the pharmacology of nafarelin and danazol.
- Sinnecker G,
Köhler S.
Sex hormone-binding globulin response to the anabolic steroid stanozolol: evidence for its suitability as a biological androgen sensitivity test.
- Gårevik N,
Strahm E,
Garle M, et al.
Long term perturbation of endocrine parameters and cholesterol metabolism after discontinued abuse of anabolic androgenic steroids.
- Lovejoy JC,
Bray GA,
Bourgeois MO, et al.
Exogenous androgens influence body composition and regional body fat distribution in obese postmenopausal women – a clinical research center study.
- Adachi M,
Takayanagi R.
Effect of anabolic steroids on osteoporosis [in Japanese].
- Dickey RP,
Taylor SN,
Curole DN.
Serum estradiol and danazol. I. Endometriosis response, side effects, administration interval, concurrent spironolactone and dexamethasone.
- Dickey RP,
Curole DN,
Taylor SN.
Estradiol target level in treating endometriosis.
- Pokras R,
Hufnagel V.
National Center for Health Statistics.
- Diwan PV,
Kulkarni DR.
Anti-inflammatory activity of nandrolone phenylpropionate.
- Forbes KL,
Dowsett M,
Rose GL,
Mudge JE,
Jeffcoate SL.
Dosage-related effects of danazol on sex hormone binding globulin and free and total androgen levels.
- Matalliotakis IM,
Neonaki MA,
Panidis DK, et al.
Three-year follow-up of [CA-125, CA 19-9, CA 15-3, SIL-2R, IL-6, IL-1a, TNF-a, sCD8 and sCD4] in a woman with severe endometriosis.
- Schmidt PJ,
Steinberg EM,
Negro PP, et al.
Pharmacologically induced hypogonadism and sexual function in healthy young women and men.
- Grow DR,
Williams RF,
Hsiu JG,
Hodgen GD.
Antiprogestin and/or gonadotropin-releasing hormone agonist for endometriosis treatment and bone maintenance: a 1-year primate study.
- Flory JD,
Matthews KA,
Sistilli CG, et al.
Short-term suppression of ovarian function and immune measures in healthy women.
- Liang SG,
Taketani Y,
Yano T,
Mizumo M.
Regulation of aromatase activity by gonadotropin-releasing hormone and its agonists in cultured rat granulosa cells [in Japanese].
- Terakawa N.
Studies on endocrine therapy of endometriosis [in Japanese].
- Jeppsson S,
Gershagen S,
Johansson ED,
Rannevik G.
Plasma levels of medroxyprogesterone acetate (MPA), sex-hormone binding globulin, gonadal steroids, gonadotrophins and prolactin in women during long-term use of depo-MPA (Depo-Provera) as a contraceptive agent.
- Tomasicchio M,
Avenant C,
Du Toit A, et al.
The progestin-only contraceptive medroxyprogesterone acetate, but not norethisterone acetate, enhances HIV-1 Vpr-mediated apoptosis in human CD4+ T cells through the glucocorticoid receptor.
- Zhang L,
Weng L.
Clinical study on women with amenorrhea after levonorgestrel intrauterine system [in Chinese].
- Pakarinen P,
Lähteenmäki P,
Rutanen EM.
The effect of intrauterine and oral levonorgestrel administration on serum concentrations of sex hormone-binding globulin, insulin and insulin-like growth factor binding protein-1.
- White T,
Jain JK,
Stanczyk FZ.
Effect of oral versus transdermal steroidal contraceptives on androgenic markers.
- Michel KG,
Huijbregts RP,
Gleason JL, et al.
Effect of hormonal contraception on the function of plasmacytoid dendritic cells and distribution of immune cell populations in the female reproductive tract.
- Strufaldi R,
Pompei LM,
Steiner ML, et al.
Effects of two combined hormonal contraceptives with the same composition and different doses on female sexual function and plasma androgen levels.
- Chetrite GS,
Pasqualini JR.
Nomegestrol acetate is an anti-aromatase agent in human MCF-7aro breast cancer cells.
- Davis SR,
Goldstat R,
Papalia MA, et al.
Effects of aromatase inhibition on sexual function and well-being in postmenopausal women treated with testosterone: a randomized, placebo-controlled trial.
- Dawood MY,
Obasiolu CW,
Ramos J,
Khan-Dawood FS.
Clinical, endocrine, and metabolic effects of two doses of gestrinone in treatment of pelvic endometriosis.
- Kodama M,
Onoue M,
Otsuka H, et al.
Efficacy of dienogest in thinning the endometrium before hysteroscopic surgery.
- Benagiano G,
Petraglia F,
Gordts S,
Brosens I.
A new approach to the management of ovarian endometrioma to prevent tissue damage and recurrence.
- Ruan X,
Seeger H,
Mueck AO.
The pharmacology of dienogest.
- Klipping C,
Duijkers I,
Remmers A, et al.
Ovulation-inhibiting effects of dienogest in a randomized, dose-controlled pharmacodynamic trial of healthy women.
- Nilsson B,
Södergård R,
Damber MG,
von Schoultz B.
Danazol and gestagen displacement of testosterone and influence on sex-hormone-binding globulin capacity.
- Heikinheimo O,
Gordon K,
Williams RF,
Hodgen GD.
Inhibition of ovulation by progestin analogs (agonists vs antagonists): preliminary evidence for different sites and mechanisms of actions.