Abstract
Glutathione (GSH), the most abundant intracellular low molecular weight thiol, has diverse physiological roles, and altered GSH status has been implicated in a number of chronic, acute, and age-related diseases, as well as the aging process itself. Its function as an antioxidant and determinant of cellular redox potential is crucial both for protection from reactive oxygen species as well as a signaling molecule involved in cellular proliferation, cell cycle regulation, and apoptosis. It is also an important thiol buffer, maintaining sulfhydryl groups in their reduced form, an additional mechanism for cellular signaling.
Glutathione has also emerged a key modulator of xenobiotic toxicity, most notably the persistent organic pollutants which are associated with many diseases of impaired metabolic activity, including diabetes, obesity, and cardiovascular disease. γ-glutamyl transpeptidase (GGT), an enzyme critical to the catabolism of GSH and its conjugates, appears to be an important biomarker for xenobiotic exposure, and for increased GSH demand. We review the physiological functions of glutathione, the limiting factors for its synthesis, as well as its clinical relevance, with particular emphasis on detoxification of environmental pollutants. We also review therapeutic approaches for enhancing GSH status.
INTRODUCTION
There is a growing recognition of the importance of glutathione (GSH) physiology, and of the number of physiological functions involving this small but critical tripeptide. Given its potent redox capabilities, and its high intracellular concentration and widespread distribution, its role as a potent defense mechanism against oxidative and electrophilic stress is well-established. Yet as our understanding of cellular signaling and regulatory pathways has developed, so has our insight into the involvement of GSH homeostasis in many other key processes, including the regulation of cellular proliferation and apoptosis and the post-transcriptional modification of proteins through S-glutathionylation, as well as the importance of GSH in the detoxification of hydroperoxides and diverse xenobiotic compounds. Additionally, GSH has been shown to be vital to mitochondrial function and maintenance of mtDNA, and may be relevant to DNA methylation.
Given the diverse roles of GSH in cellular physiology, the clinical importance of altered glutathione homeostasis is gaining well-deserved attention. Disturbances in GSH homeostasis have been implicated in neurodegenerative disorders, liver disease, cystic fibrosis, pulmonary and cardiovascular diseases, as well as the chronic age-related diseases and the aging process itself.Recent data also suggests it plays a role in metabolic and inflammatory diseases such as multiple sclerosis, the metabolic syndrome, and diabetes, at least in part by influencing the effect of multiple environmental toxins, an underappreciated yet surprisingly significant association. In this review, we will briefly describe glutathione physiology and the clinical implications of altered GSH homeostasis, as well as research on the use of various forms of glutathione as a therapeutic strategy. In light of the growing risk of persistent organic pollutants, particular attention will be given to their influence on chronic disease development, and how GSH may modulate this risk.
GLUTATHIONE BIOCHEMISTRY AND KEY PHYSICAL ROLES
Background:
Glutathione is a ubiquitous molecule, the major non-protein cellular thiol, found in millimolar concentrations in many cellular compartments and organelles, and is produced in all organs, especially the liver. It exists in both its reduced state (GSH) as well as an oxidized state (GSSG), and the relative amounts of each determine the redox status of a cell (Fig. 1.A_B). Cells at rest have a GSH/GSSG ratio exceeding 100, while in those exposed to oxidant stress the ratio drops as low as between 10 and 1, making this ratio a reasonable predictor of the cellular redox state. In addition to its role as a cellular antioxidant, because it is present in such high amounts, glutathione also functions as a thiol buffer, maintaining the sulfhydryl groups of many cellular proteins in their reduced form. Thus the GSH/GSSG ratio is important to normal functioning and is a likely contributor to cellular signaling pathways by activating or inactivating key thiol-containing enzymes.
Glutathione
Glutathione is produced exclusively in the cytosol, yet it is delivered to other cellular compartments, including the mitochondria, peroxisomes, endoplasmic reticulum, and the nucleus, as well as the extracellular space to be utilized by other tissues. For example, in the lungs, a high concentration of GSH is secreted by epithelial cells into a thin layer of fluid surrounding areas of gas exchange, and in the liver significant amounts are released into the plasma and bile. A dynamic balance is maintained between the synthesis, consumption, and transport of GSH, all of which determine its intracellular concentration. GSH is synthesized in two steps, both ATP-dependent processes, catalyzed by the enzymes GCL (glutamate-cysteine ligase, aka γ-glutamylcysteine synthetase) and glutathione synthetase, and is regenerated by six enzyme-catalyzed reactions, known as the γ-glutamyl cycle. While glutathione synthesis is controlled by multiple factors, GCL is the main determinant, because the rate-limiting step is the cellular level of the amino acid cysteine. GCL is in part regulated by GSH feedback inhibition, thus if GSH is depleted due to oxidative stress, inflammation, or exposure to xenobiotics, de novo synthesis of GSH is upregulated, as is cysteine synthesis. Cysteine is also supplied by the plasma enzyme γ-glutamyl transpeptidase (GGT) through a “salvage pathway”, and will be discussed in greater detail below. The reduction of GSSG is catalyzed by GSH reductase (GR), utilizing NADPH in the process.
PHYSIOLOGICAL FUNCTIONS
Detoxification:
All of glutathione’s reactions are via one of two mechanisms; those involving the γ-glutamyl portion of GSH, mediated by γ-glutamyl transpeptidases, with the rest involving the sulfhydryl moiety, which is responsible for the redox and nucleophilic reactions. Detoxification of both xenobiotic and endogenous compounds is a critical function of glutathione, and the one discussed here in greater detail. In addition to neutralizing endogenously generated reactive oxygen and nitrogen species, GSH is known to facilitate the plasma membrane transport of toxins by at least four different mechanisms, the most important of which is the formation of glutathione S-conjugates. GSH conjugation (followed by secretion from the cell) may be either spontaneous or catalyzed by one of several enzymes, including the GSH-transferases (GSTs), which have wide substrate diversity. This allows for reactions with a broad spectrum of both physiological metabolites (such as estrogen, prostaglandins, and leukotrienes) and xenobiotics (including acetaminophen, persistent organic pollutants, and toxic metals such as mercury, arsenic, and lead). There may also be significant interindividual differences in enzymatic capacity. For example, genetic polymorphisms in GSTs, GCL, and selenoprotein genes were shown to influence mercury body burdens among heavy fish consumers (measured as erythrocyte total mercury levels), as well as urine and hair mercury levels among dental professionals.
The associations between glutathione, chronic disease, and environmental pollutants appear to be of extraordinary significance, and yet often go unrecognized. Recent data has linked xenobiotic exposure to an increased risk for a number of cardiometabolic diseases, including cardiovascular disease, obesity, and diabetes, and it may be that depletion of glutathione levels plays a significant role in this risk, or at the very least can serve as a risk biomarker. For example, an analysis of NHANES (’99-02) data revealed that individuals with the highest serum levels of the six persistent organic pollutants (POPs) measured had a dose-dependent increase in risk for diabetes, up to an astonishing 38-fold increase. Specific POPs have been associated with a higher risk for peripheral neuropathy among those with either impaired fasting glucose or diabetes, beyond the heightened risk due to diabetes itself. Quite remarkably, in those with low POP levels, the typically robust association between diabetes and obesity was not observed in a nested case-controlled study, suggesting that obesity may exaggerate the metabolic disturbances caused by POPs (perhaps because adipose tissue provides a reservoir for fat-soluble toxins), rather than being directly responsible for the altered metabolism. This hypothesis was supported in a recent study with a 20-year follow-up, which found chronic low dose exposure to POPs predicted excess adiposity, dyslipidemia, and insulin resistance among participants without diabetes, in a U-shaped fashion (characteristic of endocrine disruption). Specific abnormalities were observed for different classes of POPs; for example, some predicted LDL levels, others triglyceride levels, and some were predictive of future BMI (Fig 2). As the authors point out, these findings may help to explain why these metabolic abnormalities occur in a cluster (such as found in the metabolic syndrome), partly because people are exposed to low doses of various POPs simultaneously. Increased risk with higher serum POP levels has also been found for the development of cardiovascular disease, hypertension, insulin resistance, and the metabolic syndrome.
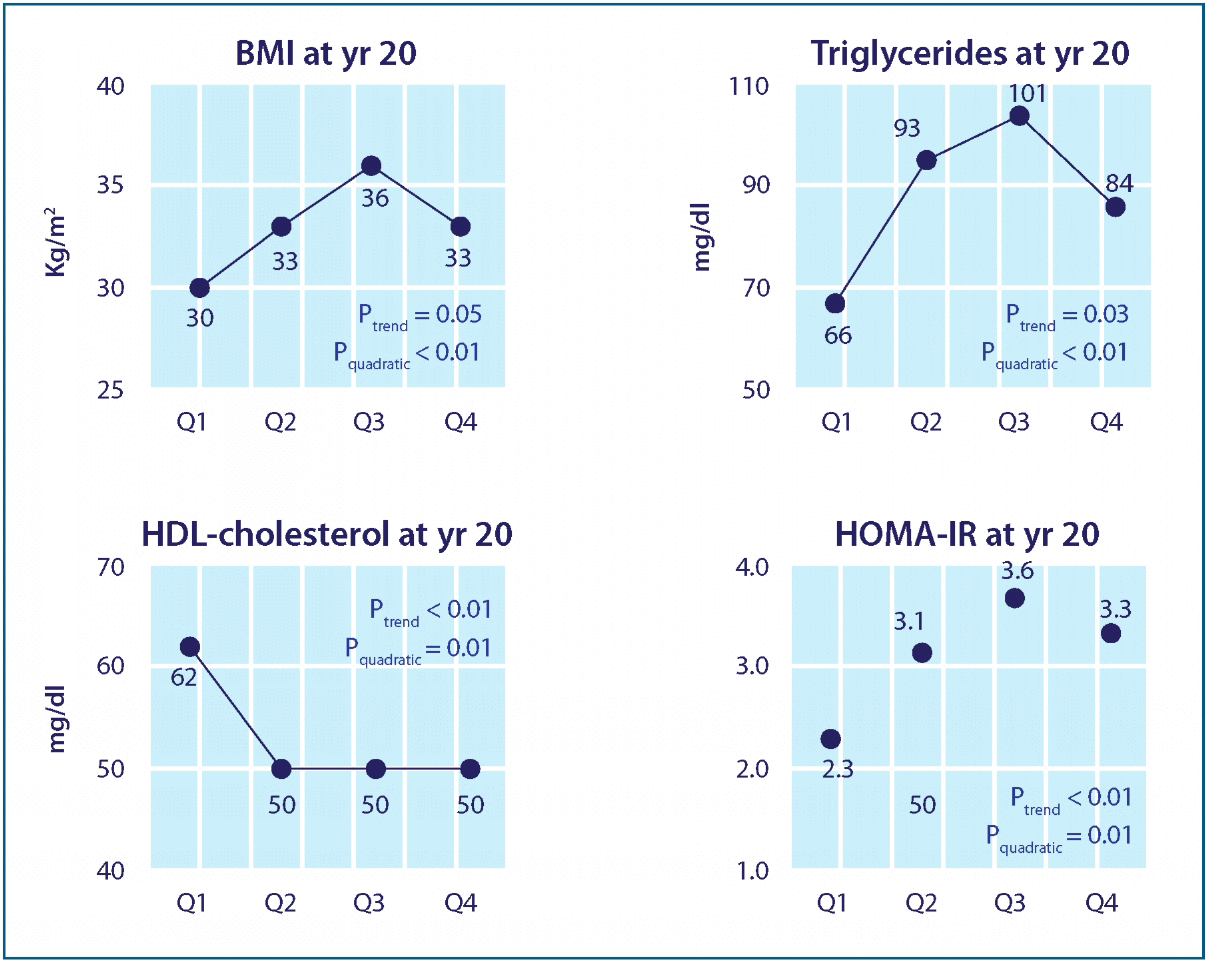
Figure 2 Effects of p,p’-DDE on BMI, dyslipidemia, and insulin resistance. Adjusted means of year 20 BMI, triglycerides, HDL-cholesterol, and HOMA-IR according to serum concentrations of p,p’-DDE at year 2. Adjusting variables were age, sex, race, BMI, triglycerides, and total cholesterol at year 2. Year 20 HDL-cholesterol and HOMA-IR were additionally adjusted for their baseline values at year 2 and year 7, respectively.
Lee DH, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR Jr. Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS One. 2011 Jan 26;6(1):e15977. Reprinted under the terms of the Creative Commons Attribution License.
GLOBAL HYPOMETHYLATION
In a commentary by Lee, et al., a hypothesis was proposed which linked chronic low dose exposure to POPs to global hypomethylation, as a consequence of GSH depletion. Global hypomethylation has been associated with cancer, atherosclerosis, as well as the aging process. Alterations in methylation patterns are also a fundamental mechanism for epigenetic modifications, allowing for transgenerational effects. In the model proposed by Lee, homocysteine is shunted away from the methionine synthesis pathway and toward the GSH synthesis pathway, creating a relative deficiency of methyl donors in order to keep up with increased demand for the glutathione needed for POP conjugation (Fig 3). The decline in GSH synthesis associated with aging combined with an increase in body burden of chemicals over a lifespan contribute to age-related hypomethylation. This is exacerbated by nutrient deficiencies, such as folate and vitamin B12, which are necessary for generating methionine. Studies in Inuit and Korean populations have supported the link between POP exposure and global hypomethylation. Interestingly, homocysteine levels have been positively associated with serum GGT, suggesting elevations in homocysteine may at least partly be due to increased shunting toward glutathione synthesis in response to increased GSH demands, though this has not been proven as of yet.
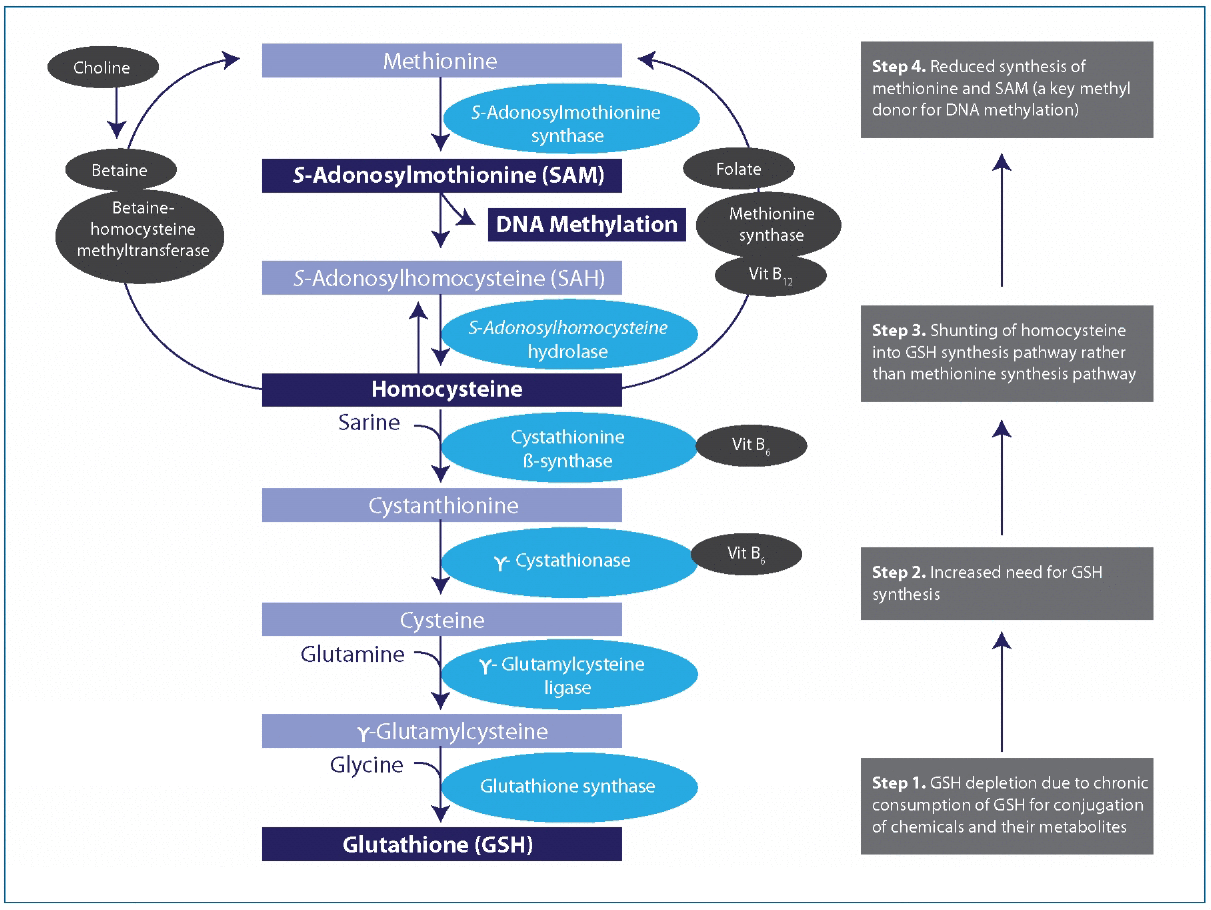
Figure 3A hypothetical unifying mechanism linking DNA hypomethylation due to chemicals and nutrient deficiency or imbalance. Vit, vitamin. DNA methylation pattern can be disturbed because of depletion of GSH when it is chronically consumed for conjugation of chemicals and their metabolites. Under usual circumstances, metabolism of homocysteine contributes to both the methionine and GSH synthesis pathways. In the presence of chemicals such as persistent organic pollutants that deplete GSH, contribution to the methionine pathway may be diminished because of greater need to synthesize GSH (numbered boxes on the right).
Lee DH, Jacobs DR Jr, Porta M. Hypothesis: a unifying mechanism for nutrition and chemicals as lifelong modulators of DNA hypomethylation. Environ Health Perspect. 2009 Dec;117(12):1799-802. Reprinted under the terms of the Creative Commons Attribution License.
GGT
As mentioned previously, GGT is a plasma enzyme expressed mainly on the apical surface of cells, and the only enzyme capable of breaking the γ-linkage found in GSH and GS H-conjugates. After further hydrolysis, the residual amino acids are taken up by the cell’s specific transporters, providing the rate-limiting cysteine through this catabolic “salvage pathway”. Increases in GGT levels, therefore, are an indicator of an increased demand for glutathione synthesis, likely due to xenobiotic exposure, an association which appears to be well-supported.
For instance, a 4-year prospective study of over 4000 healthy men found that an increase in GGT was associated with an increased risk for diabetes, even while in its normal range. Men with GGT levels < 9 U/I had the lowest risk, with a dose-dependent increase in risk at higher GGT levels, as high as 26-fold in those with levels over 50 U/I. It is worth noting that even among those men with levels between 40-50 U/I, a level considered to be in the normal range, a nearly 20-fold increase in risk was found. This has led to the hypothesis that elevations in GGT reflect increasing xenobiotic-GSH conjugation, including POPs,with preliminary evidence linking GGT levels to polycyclic aromatic hydrocarbon exposure. Associations between increases in GGT and the metabolic syndrome both fatal and non-fatal coronary heart disease (CHD) events, atherosclerosis and fatty liver, gestational diabetes,cancer, hypertension, and carotid intima-media thickness have been documented in large and well-conducted studies. Although alcohol may increase GGT levels, elevations in GGT increase risk independently of alcohol intake. If conclusively proven, this may help to explain the rapid rise is diabetes and metabolic disturbances, and offer the possibility of more specifically treating the underlying dysfunctions.
OXIDATIVE STRESS AND CELL-CYCLE REGULATION
Nuclear GSH levels also have a role in regulating cellular proliferation, and influence both telomerase activity and histone function via epigenetic control. Thus glutathione is perhaps uniquely positioned to not only provide cellular protection against oxidative and xenobiotic stress, but to also directly regulate cellular activity, including cell cycle control and induction of apoptosis in overly damaged cells. The recent shift in perspective from viewing oxidants as merely “damaging agents” to key signaling molecules is an important one, and one which places GSH homeostasis as a central mediator of these communications.
MITOCHONDRIAL FUNCTION
Considering that many chronic age-related diseases appear to have some degree of mitochondrial dysfunction as part of their etiology, it is notable that alterations in mGSH homeostasis may be a central factor in this dysfunction. For example, type 2 diabetes has well-documented mitochondrial oxidative stress, accompanied by depleted or oxidized GSH levels. Also, a substantial decrease in glutathione levels precedes the mitochondrial dysfunction and neuronal death found in Parkinson’s disease, suggesting GSH depletion may initiate the subsequent impairment.It also suggests that increasing GSH levels may have potential benefit toward preventing diseases associated with mitochondrial dysfunction.
CLINICAL RELEVANCE
Evidence that glutathione is involved in the metabolic disturbances which underlie diabetes, obesity, insulin resistance, and the metabolic syndrome continues to grow as well. In addition to the relevance of POP conjugation cited previously, hyperglycemia and hyperinsulinemia appear to directly inhibit glutathione synthesis (GCL), and the increased production of reactive oxygen species which accompanies diabetes further depletes GSH levels., Increased consumption of NADPH may also impair glutathione reductase activity. Evidence from type 1 diabetes also points to an increase in GSH utilization, which in itself was associated with poorer glucose control.The “perfect storm” of obesity, oxidative stress, inflammation, adipocyte dysfunction, and the related metabolic abnormalities may also be linked by glutathione depletion. Decreased GSH levels have been shown to activate NF-kB, further impairing mitochondrial function and creating a vicious cycle. Lastly, athletic performance may also be influenced by glutathione status, as muscle activity increases reactive oxygen and nitrogen species, utilizing GSH in the process. Muscle fatigue has been shown to be delayed by the use of N-acetylcysteine (NAC), which supports glutathione synthesis.
THERAPEUTIC INTERVENTIONS
CLINICAL EXAMPLES AND THERAPEUTIC FORMS:
N-acetylcysteine (NAC)
Improving cellular glutathione status remains a clinical challenge, though interventions which enhance glutathione synthesis have for the most part been successful, most notably N-acetylcysteine (NAC). As cited above, the amino acid cysteine is a rate-limiting factor for GSH synthesis, and a variety of both clinical trials and in-vitro/in-vivo data suggest that supplying cysteine as NAC is an effective strategy for enhancing GSH production and intracellular cysteine.Intravenous NAC has been utilized for some time to treat acetaminophen and non-acetaminophen induced acute liver failure by restoring the concentration of GSH, and has been found to be safe and effective. It has also been used successfully for the treatment of acute respiratory distress syndrome at a dose of 150mg/kg on day one followed by 50mg/kg for 3 days, with efficacy in part determined by GST polymorphisms. Although some trials have not found benefit, considerable evidence suggests it also provides renal protection when given intravenously prior to coronary angiography. In patients undergoing coronary artery bypass and/or valvular surgery, intravenous NAC was shown to reduce the incidence of post-operative atrial fibrillation. It was also shown to attenuate fatigue in endurance athletes in a small placebo-controlled trial.
Yet its use is not limited to intravenous infusions, as oral NAC has also shown to be effective for increasing glutathione levels and markers of thiol status. For example, in a randomized double-blinded trial, NAC (600mg twice per day) given with L-arginine (1200mg per day) to diabetic men with hypertension lowered blood pressure, and improved many markers of endothelial function and inflammation, including C-reactive protein, fibrinogen, and LDL-cholesterol. NAC dosed at 1g twice per day was shown to significantly improve depression in patients with bipolar disorder,with 1.2g per day improving physical performance among COPD patients in a randomized trial. A previous meta-analysis of NAC use in COPD found that it reduced the occurrence of exacerbations by roughly half over the treatment period, an effect somewhat attenuated by simultaneous steroid use, but not active smoking.Men with idiopathic infertility given 600mg per day had improvement in several semen parameters as well as plasma antioxidant status. A small pilot study of women within 5 years of menopause given 2g NAC per day suggests it may reduce bone resorption, warranting larger trials.
The ability of NAC to cross the blood brain barrier has been questioned, an important consideration for use in neurodegenerative diseases. Cysteinylglycine or γ-glutamylcysteine have been proposed as possible alternatives.In a trial of adolescents with poorly controlled type 1 diabetes, a dose of 30-45mg/kg/day NAC failed to correct several markers of glutathione status, suggesting either a higher dose or alternative therapies may be indicated in this population.A recent study of patients with poorly controlled type 2 diabetes found that dietary supplementation of the amino acids cysteine and glycine, markers of glutathione synthesis and plasma oxidative stress were both improved. As mentioned previously, these same amino acids given to elderly individuals also fully restored glutathione synthesis.
Glutathione
A number of trials have been done utilizing glutathione in various forms, with mixed efficacy. Intravenous (IV) glutathione is limited by an extremely short half-life in the plasma, and it cannot cross cell membranes intact, but must be broken down and resynthesized within the cell. Nonetheless, two IV studies have been published related to Parkinson’s disease, one open-label which found significant improvement and a slowing of the disease, and one randomized and double-blinded which found only mild symptomatic improvement. In patients with peripheral artery disease, IV glutathione was shown to improve pain-free walking distance and several markers of macro- and microcirculation in a randomized and double-blinded trial. A recent trial comparing IV glutathione to IV N-acetylcysteine found the former to be more effective in preventing contrast-induced nephropathy.
Because oral glutathione is poorly absorbed and rapidly degraded in the gut, it is not often used in clinical trials. The use of nebulized GSH was used in a small but randomized pilot trial of patients cystic fibrosis, and found to improve several clinical indicators, such as peak flow. Children with chronic otitis media with effusion given glutathione as a nasal aerosol had improvement in 67% of patients, versus only 8% of controls. A small study of children with autism spectrum disorders found that both oral lipoceutical and transdermal glutathione had some efficacy in improving plasma reduced glutathione levels.
Additional Therapies and Concerns
A number of additional therapies have been shown to either modulate GSH status or glutathione-related enzymes, though lack extensive research. Both exercise and dietary interventions have been shown to increase GSH levels, particularly moderate exercise (versus vigorous exercise) and consumption of cruciferous vegetables.Additional therapies include resveratrol, selenium, whey protein, lipoic acid,pycnogenol,spirulina,silymarin,and sulforaphane.
CONCLUSION
DISCLOSURE OF INTERESTS
REFERENCES
- Ayer A, Tan SX, Grant CM, Meyer AJ, Dawes IW, Perrone GG. The critical role of glutathione in maintenance of the mitochondrial genome.
- Lee DH, Jacobs DR Jr, Porta M. Hypothesis: a unifying mechanism for nutrition and chemicals as lifelong modulators of DNA hypomethylation.
- Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases.
- Bindoli A, Fukuto JM, Forman HJ. Thiol chemistry in peroxidase catalysis and redox signaling.
- Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis.
- Diaz Vivancos P, Wolff T, Markovic J, Pallardó FV, Foyer CH. A nuclear glutathione cycle within the cell cycle.
- Biswas SK, Rahman I. Environmental toxicity, redox signaling and lung inflammation: the role of glutathione.
- Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease.
- Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI. The central role of glutathione in the pathophysiology of human diseases.
- Ballatori N, Krance SM, Marchan R, Hammond CL. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology.
- Schläwicke Engström K, Strömberg U, Lundh T, Johansson I, Vessby B, Hallmans G, Skerfving S, Broberg K. Genetic variation in glutathione-related genes and body burden of methylmercury.
- Goodrich JM, Wang Y, Gillespie B, Werner R, Franzblau A, Basu N. Glutathione enzyme and selenoprotein polymorphisms associate with mercury biomarker levels in Michigan dental professionals.
- Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, Jacobs DR Jr. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999-2002.
- Lee DH, Jacobs DR Jr, Steffes M. Association of organochlorine pesticides with peripheral neuropathy in patients with diabetes or impaired fasting glucose.
- Lee DH, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR Jr. Low dose of some persistent organic pollutants predicts type 2 diabetes: a nested case-control study.
- Carpenter DO. Environmental contaminants as risk factors for developing diabetes.
- Lee DH, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR Jr. Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes.
- Ha MH, Lee DH, Jacobs DR. Association between serum concentrations of persistent organic pollutants and self-reported cardiovascular disease prevalence: results from the National Health and Nutrition Examination Survey, 1999-2002.
- Ha MH, Lee DH, Son HK, Park SK, Jacobs DR Jr. Association between serum concentrations of persistent organic pollutants and prevalence of newly diagnosed hypertension: results from the National Health and Nutrition Examination Survey 1999-2002.
- Lee DH, Lee IK, Jin SH, Steffes M, Jacobs DR Jr. Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999-2002.
- Lee DH, Lee IK, Porta M, Steffes M, Jacobs DR Jr. Relationship between serum concentrations of persistent organic pollutants and the prevalence of metabolic syndrome among non-diabetic adults: results from the National Health and Nutrition Examination Survey 1999-2002.
- Lee DH, Jacobs DR Jr, Porta M. Hypothesis: a unifying mechanism for nutrition and chemicals as lifelong modulators of DNA hypomethylation.
- Wilson AS, Power BE, Molloy PL. DNA hypomethylation and human diseases.
- Kim KY, Kim DS, Lee SK, Lee IK, Kang JH, Chang YS, Jacobs DR, Steffes M, Lee DH. Association of low-dose exposure to persistent organic pollutants with global DNA hypomethylation in healthy Koreans.
- Rusiecki JA, Baccarelli A, Bollati V, Tarantini L, Moore LE, Bonefeld-Jorgensen EC. Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit.
- Lippi G, Salvagno GL, Targher G, Montagnana M, Guidi GC. Plasma gamma-glutamyl transferase activity predicts homocysteine concentration in a large cohort of unselected outpatients.
- Lee DH, Ha MH, Kim JH, Christiani DC, Gross MD, Steffes M, Blomhoff R, Jacobs DR Jr. Gamma-glutamyltransferase and diabetes--a 4 year follow-up study.
- Lee DH, Steffes MW, Jacobs DR Jr. Can persistent organic pollutants explain the association between serum gamma-glutamyltransferase and type 2 diabetes?
- Lee DH, Jacobs DR Jr. Is serum gamma-glutamyltransferase a marker of exposure to various environmental pollutants?
- Lee DH, Jacobs DR. Is serum gamma-glutamyltransferase an exposure marker of xenobiotics? Empirical evidence with polycylic aromatic hydrocarbon.
- Jo SK, Lee WY, Rhee EJ, Won JC, Jung CH, Park CY, Oh KW, Park SW, Kim SW. Serum gamma-glutamyl transferase activity predicts future development of metabolic syndrome defined by 2 different criteria.
- Lee DH, Silventoinen K, Hu G, Jacobs DR Jr, Jousilahti P, Sundvall J, Tuomilehto J. Serum gamma-glutamyltransferase predicts non-fatal myocardial infarction and fatal coronary heart disease among 28,838 middle-aged men and women.
- Kozakova M, Palombo C, Paterni M, Dekker J, Flyvbjerg A, Mitrakou A, Gastaldelli A, Ferrannini E. The RISC Investigators. Fatty liver index, gamma-glutamyltransferase and early carotid plaques.
- Alanbay I, Coksuer H, Ercan M, Keskin U, Karasahin E, Ozturk M, Tapan S, Ozturk O, Kurt I, Ergun A. Can serum gamma-glutamyltransferase levels be useful at diagnosing gestational diabetes mellitus?
- Van Hemelrijck M, Jassem W, Walldius G, Fentiman IS, Hammar N, Lambe M, Garmo H, Jungner I, Holmberg L. Gamma-glutamyltransferase and risk of cancer in a cohort of 545,460 persons - the Swedish AMORIS study.
- Lee DH, Jacobs DR Jr, Gross M, Kiefe CI, Roseman J, Lewis CE, Steffes M. Gamma-glutamyltransferase is a predictor of incident diabetes and hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) Study.
- Eroglu S, Sade LE, Polat E, Bozbas H, Ulus T, Muderrisoglu H. Association between serum gamma-glutamyltransferase activity and carotid intima-media thickness.
- Fraser A, Harris R, Sattar N, Ebrahim S, Smith GD, Lawlor DA. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British Women’s Heart and Health Study and Meta-Analysis.
- Franco R, Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant.
- Filomeni G, Aquilano K, Civitareale P, Rotilio G, Ciriolo MR. Activation of c-Jun-N-terminal kinase is required for apoptosis triggered by glutathione disulfide in neuroblastoma cells.
- Marí M, Morales A, Colell A, García-Ruiz C, Fernández-Checa JC. Mitochondrial glutathione, a key survival antioxidant.
- Aquilano K, Baldelli S, Ciriolo MR. Glutathione is a crucial guardian of protein integrity in the brain upon nitric oxide imbalance.
- Pallardó FV, Markovic J, García JL, Viña J. Role of nuclear glutathione as a key regulator of cell proliferation.
- Diaz Vivancos P, Wolff T, Markovic J, Pallardó FV, Foyer CH. A nuclear glutathione cycle within the cell cycle.
- Kulinsky VI & Kolesnichenko LS. Mitochondrial glutathione.
- Ayer A, Tan SX, Grant CM, Meyer AJ, Dawes IW, Perrone GG. The critical role of glutathione in maintenance of the mitochondrial genome.
- Santos D.L, Palmeira C.M, Seiça R, Dias J, Mesquita J, Moreno A.J, Santos M.S. Diabetes and mitochondrial oxidative stress: a study using heart mitochondria from the diabetic Goto-Kakizaki rat.
- Mastrocola RRestivo F, Vercellinatto I, Danni O, Brignardello E, Aragno M, Boccuzzi G. Oxidative and nitrosative stress in brain mitochondria of diabetic rats.
- Merad-Boudia M, Nicole A, Santiard-Baron D, Saillé C, Ceballos-Picot I. Mitochondrial impairment as an early event in the process of apoptosis induced by glutathione depletion in neuronal cells: relevance to Parkinson’s disease.
- Rebrin I, Sohal RS. Pro-oxidant shift in glutathione redox state during aging.
- Sekhar RV, Patel SG, Guthikonda AP, Reid M, Balasubramanyam A, Taffet GE, Jahoor F. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation.
- Borrás C, Esteve JM, Viña JR, Sastre J, Viña J, Pallardó FV. Glutathione regulates telomerase activity in 3T3 fibroblasts.
- Catherwood M.A., Powell L.A., Anderson P., McMaster D, Sharpe P.C, Trimble E.R. ( 2002). Glucose-induced oxidative stress in mesangial cells.
- Urata Y., Yamamoto H., Goto S., Tsushima H., Akazawa S., Yamashita S., Nagataki S., and Kondo T. ( 1996). Long exposure to high glucose concentration impairs the responsive expression of g-glutamylcysteine synthetase by interleukin-1b and tumor necrosis factor-a in mouse endothelial cells.
- Brownlee M. ( 2001). Biochemistry and molecular cell biology of diabetic complications.
- Darmaun D, Smith SD, Sweeten S, Sager BK, Welch S, Mauras N. Evidence for accelerated rates of glutathione utilization and glutathione depletion in adolescents with poorly controlled type 1 diabetes.
- de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences.
- Codoñer-Franch P, Valls-Bellés V, Arilla-Codoñer A, Alonso-Iglesias E. Oxidant mechanisms in childhood obesity: the link between inflammation and oxidative stress.
- Ferreira LF, Reid MB. Muscle-derived ROS and thiol regulation in muscle fatigue.
- Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X, McKenna MJ. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals.
- Dodd S, Dean O, Copolov DL, Malhi GS, Berk M. N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility.
- Kortsalioudaki C, Taylor RM, Cheeseman P, Bansal S, Mieli-Vergani G, Dhawan A. Safety and efficacy of N-acetylcysteine in children with non-acetaminophen-induced acute liver failure.
- Soltan-Sharifi MS, Mojtahedzadeh M, Najafi A, Reza Khajavi M, Reza Rouini M, Moradi M, Mohammadirad A, Abdollahi M. Improvement by N-acetylcysteine of acute respiratory distress syndrome through increasing intracellular glutathione, and extracellular thiol molecules and anti-oxidant power: evidence for underlying toxicological mechanisms.
- Moradi M, Mojtahedzadeh M, Mandegari A, Soltan-Sharifi MS, Najafi A, Khajavi MR, Hajibabayee M, Ghahremani MH. The role of glutathione-S-transferase polymorphisms on clinical outcome of ALI/ARDS patient treated with N-acetylcysteine.
- ACT Investigators. Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography: main results from the randomized Acetylcysteine for Contrast-induced nephropathy Trial (ACT).
- Kim BJ, Sung KC, Kim BS, Kang JH, Lee KB, Kim H, Lee MH. Effect of N-acetylcysteine on cystatin C-based renal function after elective coronary angiography (ENABLE Study): a prospective, randomized trial.
- Ozaydin M, Peker O, Erdogan D, Kapan S, Turker Y, Varol E, Ozguner F, Dogan A, Ibrisim E. N-acetylcysteine for the prevention of postoperative atrial fibrillation: a prospective, randomized, placebo-controlled pilot study.
- Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X, McKenna MJ. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals.
- Ferreira LF, Campbell KS, Reid MB. N-acetylcysteine in handgrip exercise: plasma thiols and adverse reactions.
- Martina V, Masha A, Gigliardi VR, Brocato L, Manzato E, Berchio A, Massarenti P, Settanni F, Della Casa L, Bergamini S, Iannone A. Long-term N-acetylcysteine and L-arginine administration reduces endothelial activation and systolic blood pressure in hypertensive patients with type 2 diabetes.
- Berk M, Dean O, Cotton SM, Gama CS, Kapczinski F, Fernandes BS, Kohlmann K, Jeavons S, Hewitt K, Allwang C, Cobb H, Bush AI, Schapkaitz I, Dodd S, Malhi GS. The efficacy of N-acetylcysteine as an adjunctive treatment in bipolar depression: an open label trial.
- Stav D, Raz M. Effect of N-acetylcysteine on air trapping in COPD: a randomized placebo-controlled study.
- Sutherland ER, Crapo JD, Bowler RP. N-acetylcysteine and exacerbations of chronic obstructive pulmonary disease.
- Ciftci H, Verit A, Savas M, Yeni E, Erel O. Effects of N-acetylcysteine on semen parameters and oxidative/antioxidant status.
- Sanders KM, Kotowicz MA, Nicholson GC. Potential role of the antioxidant N-acetylcysteine in slowing bone resorption in early post-menopausal women: a pilot study.
- Zeevalk GD, Razmpour R, Bernard LP. Glutathione and Parkinson’s disease: is this the elephant in the room?
- Darmaun D, Smith SD, Sweeten S, Hartman BK, Welch S, Mauras N. Poorly controlled type 1 diabetes is associated with altered glutathione homeostasis in adolescents: apparent resistance to N-acetylcysteine supplementation.
- Sekhar RV, McKay SV, Patel SG, Guthikonda AP, Reddy VT, Balasubramanyam A, Jahoor F. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine.
- Sechi G, Deledda MG, Bua G, Satta WM, Deiana GA, Pes GM, Rosati G. Reduced intravenous glutathione in the treatment of early Parkinson’s disease.
- Hauser RA, Lyons KE, McClain T, Carter S, Perlmutter D. Randomized, double-blind, pilot evaluation of intravenous glutathione in Parkinson’s disease.
- Arosio E, De Marchi S, Zannoni M, Prior M, Lechi A. Effect of glutathione infusion on leg arterial circulation, cutaneous microcirculation, and pain-free walking distance in patients with peripheral obstructive arterial disease: a randomized, double-blind, placebo-controlled trial.
- Saitoh T, Satoh H, Nobuhara M, Machii M, Tanaka T, Ohtani H, Saotome M, Urushida T, Katoh H, Hayashi H. Intravenous glutathione prevents renal oxidative stress after coronary angiography more effectively than oral N-acetylcysteine.
- Bishop C, Hudson VM, Hilton SC, Wilde C. A pilot study of the effect of inhaled buffered reduced glutathione on the clinical status of patients with cystic fibrosis.
- Testa B, Testa D, Mesolella M, D’Errico G, Tricarico D, Motta G. Management of chronic otitis media with effusion: the role of glutathione.
- Kern JK, Geier DA, Adams JB, Garver CR, Audhya T, Geier MR. A clinical trial of glutathione supplementation in autism spectrum disorders.
- Rundle AG, Orjuela M, Mooney L, Tang D, Kim M, Calcagnotto A, Richie JP, Perera F. Preliminary studies on the effect of moderate physical activity on blood levels of glutathione.
- Elokda AS, Nielsen DH. Effects of exercise training on the glutathione antioxidant system.
- Lampe JW. Interindividual differences in response to plant-based diets: implications for cancer risk.
- Zhang H, Shih A, Rinna A, Forman HJ. Resveratrol and 4-hydroxynonenal act in concert to increase glutamate cysteine ligase expression and glutathione in human bronchial epithelial cells.
- Zhang W, Joseph E, Hitchcock C, DiSilvestro RA. Selenium glycinate supplementation increases blood glutathione peroxidase activities and decreases prostate-specific antigen readings in middle-aged US men.
- Micke P, Beeh KM, Buhl R. Effects of long-term supplementation with whey proteins on plasma glutathione levels of HIV-infected patients.
- Zavorsky GS, Kubow S, Grey V, Riverin V, Lands LC. An open-label dose-response study of lymphocyte glutathione levels in healthy men and women receiving pressurized whey protein isolate supplements.
- Jariwalla RJ, Lalezari J, Cenko D, Mansour SE, Kumar A, Gangapurkar B, Nakamura D. Restoration of blood total glutathione status and lymphocyte function following alpha-lipoic acid supplementation in patients with HIV infection.
- Ansar H, Mazloom Z, Kazemi F, Hejazi N. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients.
- Dvoráková M, Sivonová M, Trebatická J, Skodácek I, Waczuliková I, Muchová J, Duracková Z. The effect of polyphenolic extract from pine bark, Pycnogenol on the level of glutathione in children suffering from attention deficit hyperactivity disorder (ADHD).
- Kalafati M, Jamurtas AZ, Nikolaidis MG, Paschalis V, Theodorou AA, Sakellariou GK, Koutedakis Y, Kouretas D. Ergogenic and antioxidant effects of spirulina supplementation in humans.
- Lucena MI, Andrade RJ, de la Cruz JP, Rodriguez-Mendizabal M, Blanco E, Sánchez de la Cuesta F. Effects of silymarin MZ-80 on oxidative stress in patients with alcoholic cirrhosis. Results of a randomized, double-blind, placebo-controlled clinical study.
- Tarozzi A, Morroni F, Merlicco A, Hrelia S, Angeloni C, Cantelli-Forti G, Hrelia P. Sulforaphane as an inducer of glutathione prevents oxidative stress-induced cell death in a dopaminergic-like neuroblastoma cell line.