Abstract
Objective: Lomatium dissectum is a plant native to the Western US traditionally used in the Native American culture to treat influenza, which remains a persistent threat to human health. Evidence suggests that dysregulation of cytokines and chemokines, including CXCL10, is the primary factor leading to poor prognosis in highly pathogenic influenza infection. This study was conducted to address the hypothesis that an aqueous extract of L. dissectum root inhibits CXCL10 secretion by human bronchial epithelial cells stimulated with polyinosinic:polycytidylic acid (poly i:c), a synthetic analog of viral dsRNA.
Design: BEAS-2B cells treated with poly i:c were exposed to L. dissectum root aqueous extract simultaneously or at 2 h intervals up to 8 h post-stimulation. Supernatants were harvested at 24 h and enzyme-linked immunosorbent assay (ELISA) performed to determine CXCL10 concentrations.
Results: L. dissectum root aqueous extract at 1 µg/mL significantly inhibited CXCL10 secretion (P=0.043, Anova, Tukey HSD) and demonstrated maximal inhibition 6 h post poly i:c exposure. MTT cytotoxicity assay results suggest that this inhibitory effect was not due to extract-induced cytotoxicity.
Conclusion: The observation that L. dissectum extract inhibits CXCL10 secretion provides a plausible mechanism for the efficacy of L. dissectum in influenza treatment reported in ethnobotanical studies and case reports. L. dissectum may reduce morbidity and mortality associated with influenza and merits further research.
INTRODUCTION
Highly pathogenic influenza A remains a persistent threat to human health. Evidence suggests that vaccine coverage would likely be inadequate during the first six months of a pandemic. Vaccine efficacy is limited in pandemics, as the causative strain of virus cannot be known until the pandemic begins and according to the WHO, “Global manufacturing capacity for influenza vaccines is limited, inadequate and not readily augmented”. This leaves antiviral therapy as a critical treatment needed for effective response to highly pathogenic influenza pandemics.
There are currently four antiviral drugs licensed for use to treat influenza, which include the adamantanes, adamantadin and rimantadin, and the neuraminidase inhibitors, oseltamivir and zanimivir. Resistance of influenza viruses to these agents has proven problematic. Since 2006, the CDC has recommended against the use of adamantanes due to resistance. Furthermore, resistance to oseltamivir between the 2007–2008 and 2008–2009 influenza seasons increased from 19.9% to 99.6%. As resistance to these antiviral medications limits their usefulness, novel treatment approaches, such as those that modulate immune responses to influenza, need to be explored.
The morbidity and mortality from highly pathogenic influenza infection is primarily caused by cytokine and chemokine dysregulation that may occur during the immune response to influenza and not by the virus itself. In recent studies of humans infected with highly pathogenic H5N1 influenza, high levels of the chemokine CXCL10 were correlated with poor prognosis and high viral load. CXCL10 is a chemo-attractant for T lymphocytes, NK cells, and macrophages that express its ligand, CXCR3. High levels of CXCL10 may be responsible for excessive pulmonary macrophage infiltration. Overproduction of CXCL10 has also been demonstrated in animal and cell culture models of influenza. Thus, therapies that target the immune response and inhibit CXCL10 production are ideal candidates for investigation. One potential phytotherapeutic agent with ethnobotanical evidence of use in the treatment of influenza is Lomatium dissectum.
Lomatium dissectum aqueous root preparations have been traditionally used in the treatment of respiratory tract infections. This Apiaceae species is native to western North America and was an important medicine for the peoples within its range. During the 1918 influenza pandemic, L. dissectum was hailed as curative of influenza and influenza-associated pneumonia. Currently used by naturopathic physicians to treat respiratory tract infections, root extracts from this plant merit further study.
Because an aqueous extract of L. dissectum root was the form traditionally used to prevent influenza-associated pneumonia, and CXCL10 has been shown to correlate with poor prognosis in influenza, this study examined the hypothesis that L. dissectum inhibits CXCL10 in an in vitro model of viral infection. BEAS-2B human bronchial epithelial cells treated with the synthetic double stranded RNA (dsRNA) polyinosinic:polycytidylic acid (poly i:c) is an accepted model for influenza A infection. Poly i:c triggers the immune response by binding to the toll-like receptor 3 (TLR3), a cytoplasmic receptor that binds dsRNA ligands not regularly expressed in mammalian cytoplasm. In response to poly i:c, the BEAS-2B human bronchial cell line secretes CXCL10. Inhibition of CXCL10 would suggest a novel action of L. dissectum in preventing influenza-related pathogenesis.
METHODS
CELLS AND CELL CULTURE
BEAS-2B human bronchial epithelial cells originally from the American Type Culture Collection (Manassas, VA) were maintained in a humidified incubator at 37°C and 5% CO2 in DMEM-F12 media (Invitrogen, Grand Island, NY) supplemented with 5% heat-inactivated FBS, 100 U/mL penicillin (Corning Life Sciences, Tewksbury MA), 100 μg/mL streptomycin (Corning), and 2 mM L-glutamine (Corning).
LOMATIUM DISSECTUM ROOT AQUEOUS EXTRACT
L. dissectum root (25 g) obtained from Oregon’s Wild Harvest (Sandy, OR; lot number LOM-03073w-WMJ) was decocted in 500 mL distilled water, which was reduced by 50%, and an additional 250 mL of distilled water was added. This decoction was reduced to 375 mL. The final aqueous extract was filter sterilized, and aliquots frozen at −20°C until time of assay. A final concentration of 0.16 µg/µL was determined using a Mettler Toledo HR 73 Moisture Analyzer.
MTT ASSAY:
To assess the cytotoxicity of the L. dissectum aqueous extract, a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was conducted. BEAS-2B cells were seeded in inner wells of 96 well plates. A standard curve of BEAS-2B cells in serial dilution from 30,000 to 469 cells/mL was included. The remaining wells were seeded at a density of 30,000 cells/mL and plates incubated for 24 h. Media was removed and fresh media (100 μL) added to cell standard wells. Test wells (six replicates per treatment) were treated with a serial dilution of 1:1 L. dissectumextract:media resulting in final concentrations ranging from 80 µg/mL to 1.25 µg/mL. After 24 h incubation, wells were aspirated and washed twice with 100 µL/well PBS. MTT solution was added and plates incubated for 3 h. Wells were aspirated and 100 µL/well dimethly sulfoxide (DMSO) added. Absorbance at 540 nm (OD540) was determined using a Molecular Devices Spectra Max Plus 384 plate reader.
L. DISSECTUM TREATMENT OF BEAS-2B CELLS
BEAS-2B cells seeded in 24 well plates at a density of 15,000 cells/mL were incubated for 24 h. Media was aspirated and replaced with either media alone or media with 1 μg/mL poly i:c (Sigma Aldrich). Treatment with L. dissectum root aqueous extract was either immediately after poly i:c exposure or at various time points thereafter (timed response). Cells were incubated for 24 h after stimulation and the supernatant collected and centrifuged at 1400 rpm for 5 min. Samples were used immediately in an enzyme-linked immunosorbent assay (ELISA) or stored at −20°C until ELISA, conducted within 30 days of supernatant harvest.
L. dissectum extract was tested at concentrations ranging from 1 to 10 μg/mL and for timed response assay, at 1 µg/mL. Sterile PBS (Invitrogen) was used as a control. Triplicate wells of poly i:c stimulated BEAS-2B cells were treated with L. dissectum aqueous extract either concurrently with poly i:c stimulation, or post poly i:c stimulation (timed response) at 2 h intervals up to 8 h.
ELISA
A for CXCL10 was performed using the human CXCL10 ELISA kit (R&D Systems, Minneapolis, MN) following manufacturer’s instructions. CXCL10 concentrations of triplicate supernatant samples were determined by extrapolation from a standard curve after determining absorbance (OD450–OD570) using a Molecular Devices Spectra Max Plus 384 plate reader and SoftMaxPro 5.4 software.
STATISTICS
Data were reported as mean ± standard deviation. Comparisons among treatment conditions were done using analysis of variance (ANOVA) followed by a post hoc test for multiple comparisons (Tukey Honest Significant Difference). All statistical analyses were performed using PASW statistics software. A P-value <0.05 was considered significant.
RESULTS
MTT ASSAY:
No significant enhancing or inhibitory effect on proliferation of BEAS-2B cells was observed with treatment of L. dissectum at 40–1.25 µg/mL. A cytotoxic effect was observed at the highest L. dissectum concentration tested, 80 µg/mL (Figure 1).
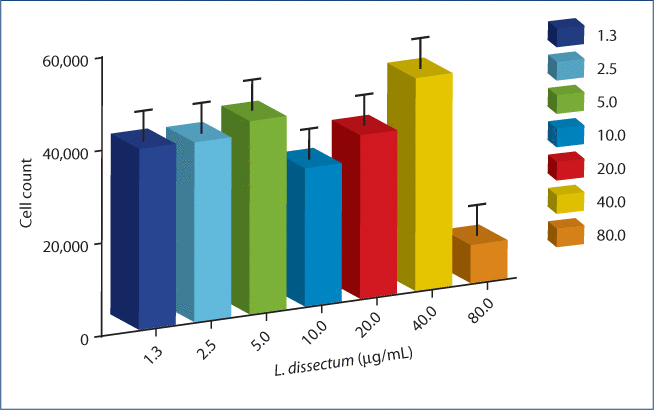
Figure 1:The cytotoxic effect of L. dissectum extract on poly i:c stimulated BEAS-2B human bronchial epithelial cells.
BEAS-2B cells were seeded at 30,000 cells/mL for 24 h. Media was replaced with media containing 1 µg/mL poly i:c and L. dissectum extract (1.3–80 μg/mL) in 6 wells per treatment condition. After 24 h incubation, MTT was added, cells incubated for another 3 h and MTT solvent DMSO added. Plates were shaken for 15 min and read on a microplate reader at OD540. No cytotoxic effect was observed at 40 µg/mL or lower L. dissectum concentration.
EFFECT OF L. DISSECTUM ON CXCL10 SECRETION
Poly i:c stimulation of BEAS-2B cells induced significant CXCL10 secretion above constitutively expressed levels observed in the media control (Figure 2). L. dissectum (1 µg/mL) treatment concurrent with poly i:c stimulation resulted in significant inhibition (P=0.043) of CXCL10 secretion by BEAS-2B cells compared to poly i:c stimulation alone. This treatment decreased CXCL10 to a concentration that was not significantly different than that of the media control. Concentrations of 5 and 10 µg/mL L. dissectum led to a non-statistically significant trend in decreased CXCL10 secretion compared to poly i:c stimulation. Furthermore, treatment of unstimulated BEAS-2B cells with L. dissectum (1 µg/mL) resulted in significant inhibition of CXCL10 compared to unstimulated media control (data not shown).
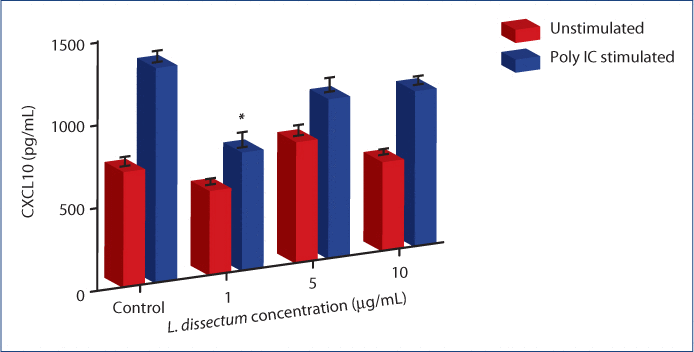
Figure 2:L. dissectum decreases CXCL10 production by poly i:c stimulated BEAS-2B human bronchial epithelial cells.
BEAS-2B cells were seeded at 15,000 cells/mL and incubated for 24 h. Media was replaced with media containing 1 μg/mL poly i:c and L. dissectum at 1, 5 or 10 µg/mL. Cells were incubated for 24 h, supernatants harvested and assayed for CXCL10 secretion by ELISA. CXCL10 secretion was significantly inhibited compared to poly i:c alone (P=0.043) at a concentration of 1 μg/mL. *Significantly different (P=0.043) than poly i:c stimulation
TIMED RESPONSE OF L. DISSECTUM ON CXCL10 SECRETION
Cells were treated with L. dissectum (1 µg/mL) at either 2, 4, 6, or 8 h post stimulation with poly i:c (Figure 3). Treatment at 2, 4 and 6 h resulted in significant inhibition (P<0.001) of CXCL10 secretion compared to poly i:c stimulated BEAS-2B cells. Treatment at 6 h post-stimulation produced maximal inhibition, resulting in a CXCL10 concentration that was not significantly different than unstimulated media control. L. dissectum treatment at 8 h post poly i:c stimulation resulted in insignificant inhibition of CXCL10 compared to poly i:c stimulation alone.
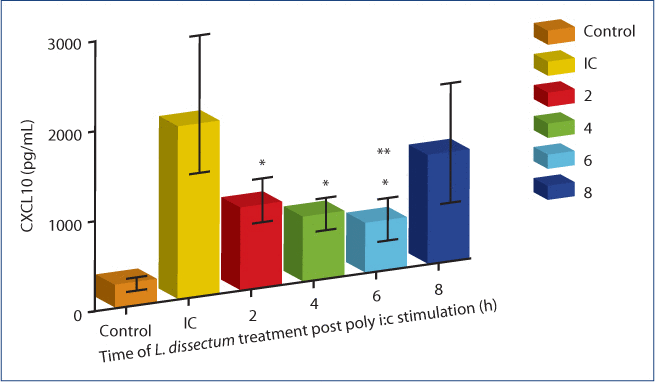
Figure 3:The effect of L. dissectum treatment time on CXCL10 secretion by BEAS-2B cells.
BEAS-2B cells were seeded at 15,000 cells/mL and incubated for 24 h. Media was replaced with media containing poly i:c (1 µg/mL). L. dissectum extract (1 µg/mL) was added at 2, 4, 6, or 8 h post poly i:c stimulation. Cells were incubated for 24 h post poly i:c addition, supernatants harvested and assayed for CXCL10 secretion by ELISA. L. dissectum treatment at 2, 4, and 6 h post poly i:c stimulation significantly inhibited CXCL10 secretion (P<0.001). L. dissectum treatment at 6 h resulted in CXCL10 secretion that was not significantly different than unstimulated media control. *Significantly different (P<0.001) than poly i:c stimulation; **Not significantly different than media control.
DISCUSSION
L. dissectum root has traditionally been used in the treatment of respiratory tract infections, including influenza A infection. Despite its rich ethnobotanical heritage, L. dissectum has been insufficiently studied. This is the first report of an inhibitory effect of an aqueous L. dissectum root extract on CXCL10 secretion, a chemokine implicated in the pathogenesis of influenza A infection. This inhibitory effect was not due to cytotoxicity of the extract on BEAS-2B cells, since L. dissectum was not cytotoxic at the concentrations that decreased CXCL10 secretion. L. dissectumsignificantly inhibited CXCL10 secretion when added up to 6 h post poly i:c stimulation, suggesting that aqueous L. dissectum root extract may be useful as treatment during influenza virus infection. Early and excessive production of CXCL10 is also associated with poor prognosis in other conditions such as ARDS, hepatitis, SARS-CoV infection, inflammatory bowel disease, and other autoimmune conditions. L. dissectum warrants further investigation as a novel treatment for the prevention of influenza-associated morbidity and mortality and for other diseases associated with CXCL10 dysregulation.
To date, there have been few investigations of the biological effects of L. dissectum extracts. The dichloromethane fraction contains water soluble tetronic acids, flavonoids and three coumarin glycosides, including an apiose glycoside. The tetronic acids are antimicrobial and both the aqueous and volatile oil fractions of L. dissectum root have demonstrated widespread antibacterial activity. The purported effect of L. dissectum in the treatment of respiratory tract infections may be due to a combination of direct antibacterial effects and immunomodulatory effects. Given that the effect of L. dissectum on CXCL10 was the focus of this study, more in-depth investigations of immunomodulatory effects of the aqueous root extract and its constituents are needed.
This preliminary investigation is limited in that the results may be due to unidentified microbially-derived elements or other impurities in the root extract. Although the raw L. dissectum starting material tested negative for Escherichia coli, the final extract was not tested for endotoxin or other microbial components. Further, because no information is available about the bioavailability of L. dissectum extracts, the physiological relevance of the concentrations tested is unknown. Although significant inhibition of CXCL10 at 1 µg/mL was observed up to 6 h post poly i:c stimulation, the higher concentrations tested showed an insignificant trend toward decreased CXCL10 secretion.
L. dissectum has been regarded as safe in the historic literature, with a rash being the main reported side effect and those allergic to plants in the Apiaceae family are advised against its use. Nausea from ingestion of large amounts has been reported, although one of its traditional uses was for stomach and other internal disorders. L. dissectum is listed as “at risk” by United Plant Savers and although its ecological status is debated among experts, conscientious harvesting is necessary to ensure the survival of native stands of L. dissectum. Establishment of L. dissectum plant fields is possible but extended seed stratification is required for successful propagation and established plants typically do not produce seeds and flowers until the fourth year of production. Contacting local experts or visiting resources such as The Northeast School of Botanical Medicine website for wildcrafting references is recommended before harvesting.
ACKNOWLEDGMENTS
We wish to thank Lisa Price, ND and Masa Sagasawa, ND for their technical assistance. The authors have no conflict of interest to disclose.
DISCLOSURE OF INTERESTS
Dr. Zamechek and Dr. Wenner have nothing to disclose.
REFERENCES
- Maines TR, Szretter KJ, Perrone L, et al. Pathogenesis of emerging avian influenza viruses in mammals and the host innate immune response.
- Webster RG. Influenza: an emerging disease.
- WHO. World Health Organization Epidemic and Pandemic Alert and Response. Pandemic Flu Frequently asked questions. [Internet]. [5/2/2009:5/18/2009] <http://www.who.int/csr/disease/swineflu/frequently_asked_questions/vaccine_preparedness/en/index.html> .
- Webby RJ, Webster RG. Are we ready for pandemic influenza?
- Subbarao K, Murphy BR, Fauci AS. Development of effective vaccines against pandemic influenza.
- WHO. Pandemic influenza vaccines: current status [Internet] [9/24/2009;10/28/2009]. <http://www.who.int/csr/disease/swineflu/notes/pandemic_influenza_vac?cines_20090924/en/index.html> .
- Guralnik M, Rosenbloom RA, Petteruti MP, Lefante C. Limitations of current prophylaxis against influenza virus infection.
- Hayden FG, Pavia AT. Antiviral management of seasonal and pandemic influenza.
- Wu JT, Leung GM, Lipsitch M, Cooper BS, Riley S. Hedging against antiviral resistance during the next influenza pandemic using small stockpiles of an alternative chemotherapy.
- CDC. Background: Interim recommendations for the use of influenza antiviral medications in the setting of oseltamivir resistance among circulating influenza A (H1N1) viruses, 2008-09 Influenza Season [Internet]. [12/12/2009; 4/13/2010] <http://www.cdc.gov/flu/professionals/antivirals/background.htm> .
- CDC. Weekly Influenze Surveillance Report [Internet]. [11/7/2011;6/4/2009] <http://www.cdc.gov/flu/weekly/weeklyarchives2009-2010/09-10summary.htm> .
- Santibanez S, Fiore AE, Merlin TL, Redd S. A primer on strategies for prevention and control of seasonal and pandemic influenza.
- de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia.
- Peiris JS, Yu WC, Leung CW, et al. Re-emergence of fatal human influenza A subtype H5N1 disease.
- Baskin CR, Bielefeldt-Ohmann H, Tumpey TM, et al. Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus.
- Cameron CM, Cameron MJ, Bermejo-Martin JF, et al. Gene expression analysis of host innate immune responses during Lethal H5N1 infection in ferrets.
- Kobasa D, Jones SM, Shinya K, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus.
- Jassim SA, Naji MA. Novel antiviral agents: a medicinal plant perspective.
- Weinstock DM, Zuccotti G. The evolution of influenza resistance and treatment.
- Moore M.
- NaturalStandard. Lomatium Professional Monograph [Internet]. [6/4/2009] <http://www.naturalstandard.com/naturalstandard/monographs/monoframeset.asp?monograph=/monographs/herbssupplements/desertparsley.asp&patientVersion=/monographs/herbssupplements/patient-desertparsley.asp> .
- Chamberlin RV.
- Moerman DE.
- Moerman DE.
- Turner NJ, Royal British Columbia Museum.
- Krebbs E. Indian remedy for influenza. In: Editor GR, ed.
- Mitchell WA, Bastyr JB.
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3.
- Guillot L, Le Goffic R, Bloch S, et al. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus.
- Matsukura S, Kokubu F, Kurokawa M, et al. Synthetic double-stranded RNA induces multiple genes related to inflammation through Toll-like receptor 3 depending on NF-kappaB and/or IRF-3 in airway epithelial cells.
- Takeuchi O, Akira S. Innate immunity to virus infection.
- Xagorari A, Chlichlia K. Toll-like receptors and viruses: induction of innate antiviral immune responses.
- Taima K, Imaizumi T, Yamashita K, et al. Expression of IP-10/CXCL10 is upregulated by double-stranded RNA in BEAS-2B bronchial epithelial cells.
- Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, Schleimer RP. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism.
- Song M, Kim YJ, Ryu JC. Phospholipidosis induced by PPARgamma signaling in human bronchial epithelial (BEAS-2B) cells exposed to amiodarone.
- Askarieh G, Alsio A, Pugnale P, et al. Systemic and intrahepatic interferon-gamma-inducible protein 10 kDa predicts the first-phase decline in hepatitis C virus RNA and overall viral response to therapy in chronic hepatitis C.
- Falconer K, Askarieh G, Weis N, Hellstrand K, Alaeus A, Lagging M. IP-10 predicts the first phase decline of HCV RNA and overall viral response to therapy in patients co-infected with chronic hepatitis C virus infection and HIV.
- Katoh S, Fukushima K, Matsumoto N, et al. Accumulation of CXCR3-expressing eosinophils and increased concentration of its ligands (IP10 and Mig) in bronchoalveolar lavage fluid of patients with chronic eosinophilic pneumonia.
- Turner JE, Steinmetz OM, Stahl RA, Panzer U. Targeting of Th1-associated chemokine receptors CXCR3 and CCR5 as therapeutic strategy for inflammatory diseases.
- Tang NL, Chan PK, Wong CK, et al. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome.
- VanWagenen BC, Huddleston J, Cardellina JH, 2nd. Native American food and medicinal plants, 7. Antimocrobial Tetronic Acids From Lomatium dissectum.
- VanWagenen BC, Huddleston J, Cardellina JH, 2nd. Native American food and medicinal plants, 8. Water-soluble constituents of Lomatium dissectum.
- Carlson HJ, Douglas HG. Antibiotic agents separated from the root of lace-leaved Leptotaenia.
- McCutcheon AR, Ellis SM, Hancock RE, Towers GH. Antibiotic screening of medicinal plants of the British Columbian native peoples.
- Moerman DE.
- USDA. Lomatium Dissectum Plant Profile. [Internet]. [1/28/2011;9/8/2013] <http://plants.usda.gov/core/profile?symbol=LODI> .
- UPS UPS. Species At-Risk. [Internet]. [5/08/2012;9/7/2013] <httphttp://www.unitedplantsavers.org/content.php/121-species-at-risk> .