Abstract
Plant based natural products have been proposed as alternatives to the use of hormones for the treatment of menopausal symptoms, which has been associated with an increased risk of breast cancer and coronary artery disease. Specific herbal compounds such as Thai Kudzu root (Pueraria mirifica) have shown beneficial effects in the treatment of peri-menopausal symptoms and menopausal-related osteoporosis when used as an adjunct or as a single medicine. Pueraria mirifica has been used in conjunction with Vitex Berry (Vitex agnus-castus), Red Clover (Trifolium pratense) Sage (Salvia officinalis and S. miltiorrhiza), and Black Cohosh (Actaea racemosa), with the optional addition of natural progesterone and soy milk. Clinically, these compounds require careful dosing and are associated with variable times to efficacy. No known drug interactions exist, although research on the effects of phytoestrogens is limited. Phytoestrogens have not been associated with increased risk of cancer and therefore provide a safe alternative to standard treatment modalities.
AARM REFERENCE REVIEWS:These are AARM reference reviews in which clinicians can obtain a quick overview of understanding with respect to how herbs, nutrients, and hormones can be used effectively in clinical practice. The dosages recommended are based on therapeutic results. Side effects that have not been seen in clinical experience or found in clinical studies but only been theorized have been identified and depicted as Unsubstantiated Theoretical Concerns. Since many nutrients and herbs on the market have not gone through double-blind studies but have been used extensively in clinical settings for hundreds, and for some thousands of years, these reference reviews have attempted to give a unified opinion on how to use these herbs effectively. The opinions expressed are from different professors and experts in the field of nutritional and botanical medicine. Some information is based on personal opinions and can not be quantified as fact. However, if clinicians only restricted themselves to what is fully proven by double-blind studies, the art of healing and restoring health with nutrients and herbs would be lost. |
CLINICAL IMPLICATIONS
The therapeutic use of hormones to relieve menopausal symptoms has been linked to an increased risk of breast cancer as well as coronary artery disease. Therefore, specific herbs (e.g. Thai Kudzu Root (Pueraria mirifica)) may be beneficial in the management of peri-menopausal hot flashes, vaginal atrophy, and loss of bone density post-menopause. Further, these herbs are indicated as they are not implicated with an increased risk of cancer and offer a safe alternative to standard treatment modalities.
PRIMARY INDICATION:
Peri-menopausal symptoms and menopausal-related osteoporosis
ADJUNCTIVE OR STAND-ALONE TREATMENT:
Adjunctive or Stand-Alone
DOSE OF BIOACTIVE CONSTITUENTS:
Pueraria mirifica (whole plant extracts 400-900 mg per day; containing 2.5 mg of Puerarin. Synergistic Herbal Formula: Thai Kudzu root (Pueraria mirifica, Vitex Berry (Vitex agnus-castus), Red Clover (Trifolium pratense) Sage (Salvia officinalis and S. miltiorrhiza), Black Cohosh (Actaea racemosa). Natural progesterone and the dietary addition of soy milk are additional options to relieve specific symptoms.
DURATION OF TIME FOR EFFICACY:
Physicians have noted that therapeutic results occur more reliably at higher doses; at lower doses, results have been disappointing. At correct dosage, results can be seen within a month.
LAB TEST TO ASSESS EFFICACY:
None known
TIME TO CLINICAL EFFICACY:
Variable
DRUG INTERACTIONS AND CAUTIONS:
These herbs can be safely consumed when used appropriately
UNSUBSTANTIATED THEORETICAL CONCERNS:
Pueraria may increase the anticoagulant effects and bleeding risk of anticoagulant and anti-platelet medications. Pueraria may inhibit Tamoxifen from binding to some estrogen receptors, and may competitively inhibit the effects of oral contraceptives and estrogen therapy. Pueraria may also lower blood glucose levels and may have additive effects when used with glucose lowering medications and supplements, increasing the risk of hypoglycemia in some patients. No clinical or scientific evidence has validated any of these concerns.
DISCUSSION
Embryologic development of the breasts, prostate and reproductive organs involves both estrogens and androgens. Exposure to exogenous estrogens in the neonatal period such as hormonally active synthetic chemicals, may increase the risk of hormonally-related cancers later in life. Similarly, steroidal metabolism and signaling may be disrupted early in life through exposure to hormonally-active synthetic chemicals. This phenomenon is referred to as “endocrine disruption.” Conversely, natural plant-based compounds such as phytoestrogens may reduce the chances of hormone-related reproductive cancers later in life. Phytoestrogens may mitigate the severity of endocrine disruptors, and offer positive hormonal influences on the breast and prostate. Furthermore, genes that control cellular and hormonal receptor quantities and response are also shown to be unfavorably influenced by exogenous chemicals and favorably influenced by phytoestrogens.
This review suggests that phytoestrogens offer a positive influence (as early as in utero) on hormonal balance, when included in the diet on a regular basis. Phytoestrogens also appear to be valuable in the treatment protocols for breast and prostate cancers. Although phytoestrogens can exert weak estrogenic effects themselves, there is emerging evidence that such hormonal actions do not stimulate cancer cell growth, but rather mitigate (or even block) endogenous hormone effects by: 1) inhibiting hormone receptors, 2) modulating enzyme systems that process hormones, and 3) interact with hormone-regulating genes.
Standard doses of specific phytoestrogens include Pueraria Extract (400 mg twice per day), or Genistein (50 mg twice per day). Side-effects of high-dose soy extract containing phytoestrogens have been implicated in causing a mild increase in TSH for post-menopausal women. However, this effect was only present in patients with low iodine levels; to date, no adverse interactions between phytoestrogens and prescription drugs have been published.
In animal models (at andropause and middle-age, when thyroid function may decline) in many species—both daidzein and genistein were reported by Sosic-Jurjevic et al to induce microfollicular changes in the thyroid and depress thyroid function enough to produce a measureable increase in TSH.However, other researchers have suggested substances other than soy isoflavones are responsible for the thyroid function suppression. In animals, Doerge et al suggest that pre-existing iodine deficiency is necessary for soy to exert a thyrosuppressive effect.
PHYTOESTROGENS AS SELECTIVE ESTROGEN-RESPONSE MODIFIERS:
Alpha and beta estrogen receptors oppose and balance one another. While alpha subtype receptors direct cellular proliferation, beta estrogen receptors direct cellular differentiation and apoptosis. Research to develop specific estrogen receptor subtypes found in specific tissue types as a means to improve the treatment of estrogen-dependent cancers and disease is currently underway. These therapeutic agents are referred to as Selective Estrogen Response Modifiers or SERMs.

Actaea racemosaof the Ranunculaceae family
© Steven Foster Group, Inc.
Genistein (an isoflavone type of phytoestrogen common in legumes) is a beta subtype estrogen receptor agonist, and thus can be considered a natural SERM. Genistein is one of the most well-researched and active isoflavone phytoestrogens. Genistein may affect gene expression due to direct activity at estrogen receptors. Studies have shown that Genistein may improve the response of estrogen positive cancers to radiation, and inhibit prostate cancer cell growth. Genistein also positively influences steroid-producing genes, and activates several genes associated with tumor suppression.Therapeutic supplementation with isoflavones in dosages that exceed typical dietary amounts may slow the progression of prostate cancer without any noticeable side effects or toxicity. In one study, male patients diagnosed with prostate cancer were administered isoflavones in the form of soy milk to deliver a standardized amount of Genistein three times per day (for a total of 141 mg per day) for one year. For nearly all of the research subjects, serum equol was increased; for many men the upward trend of PSA levels was either stabilized or even reversed. Note: Equol is a metabolite of daidzein produced in the gut, and a high level has been associated with health benefits.
THE AMPHOTERIC ACTION OF PHYTOESTROGENS:
When phytoestrogens bind to estrogen receptors, they can act as either agonists or antagonists. This activity and ligand affinity may render phytoestrogens the ideal SERMs. For example, isoflavones might act as weak agonists in situations of low estrogen in the body, yet might also reduce estrogenic stimulation. This dual action of phytoestrogens—to both offer estrogenic support and reduce excessive estrogen stimulation—is referred to as “amphotericism” by many clinical herbalists. Inclusion of isoflavones and other phytoestrogens either in the form of herbal supplements or in the diet is believed to offer many health benefits such as protection against hormone-related cancers. In a cellular assay, synthetic phytoestrogen-like compounds were tested, and interestingly displayed no such amphoteric action. In terms of phytoestrogens and hormonal enzyme systems, the enzymes affected by phytoestrogens include aromatase, dehydrogenase, reductase and sulphotransferase enzyme; in turn, all of these enzymes may affect hormonally-related cancers.
Sulfotransferases: Sulfotransferases are Phase II detoxifying (conjugation) enzymes. Sulfotransferases add sulfur to estrogens, rendering them less active; this occurs because sulfated estrogens do not bind to estrogen receptors. Genistein, Quercitin, and Resveratrol have been shown to promote the activity of sulfotransferases.
Dehydrogenases: 17-beta dehydrogenase enzymes are responsible for oxidizing steroids, making them less active and reducing the overall amount of active hormone circulating in the bloodstream. Breast cancer, prostate cancer and endometriosis are thought by some to be steroid-dependent diseases that display imbalances of dehydrogenase enzymes and the hormones they regulate. Many phytoestrogens (including flavonoids, coumarins, and coumestans) have been noted to inhibit 17-beta dehydrogenase.
5 Alpha Reductase Inhibitors: 5-alpha-reductase is the enzyme that is responsible for the conversion of testosterone into its active form, dihydrotestosterone (DHT). Since DHT has greater stimulatory and proliferative effects on the prostate gland compared to testosterone, prostate enlargement and cancer treatment may include inhibition of this enzyme. Finasteride (Proscar) is a synthetic pharmaceutical agent used for this purpose. High fat diets (which affect the genes that control 5-alpha-reductase in the prostate) stimulate prostate enlargement; this may be mitigated by dietary enhancement with Genistein. Some botanicals that inhibit 5-alpha-reductase activity are Saw Palmetto (Serenoa repens), Pygeum Bark (Pygeum africanum), Nettle Root (Urtica dioica) and Green Tea (Camellia sinensis) catechins. In animal models, Serenoa repens had been shown to inhibit tumorigenesis and induce apoptosis of prostate cancer cells. Habib et al demonstrated that Serenoa inhibits 5-alpha-reductase and reduces circulating levels of active testosterone in humans. Other sources claim that Serenoa is weak in its ability to block 5-alpha reductase, for example Rhodes et al. Interestingly, Habib and colleagues also found that Serenoa repens (10 μg/ml) could reduce enzyme activity by 72% without interfering with secretion of prostate specific antigen (PSA).
Aromatase Inhibitors: Aromatase enzymes are abundant in peripheral lipid cells, convert circulating androgens into active estrogens, and have been found in high amounts in prostate cells. Research studies are investigating the effects of aromatase inhibitors as breast and prostate cancer therapy since aromatase inhibitors suppress circulating estrogen levels and, thus, may inhibit the proliferation of estrogen dependent cancer cells.
Phytoestrogens, especially those containing a coumarin backbone (rather than the isoflavone or steroid ring structures) have been shown to inhibit aromatase, thereby reducing circulating estrogen levels by approximately 96-98%. Additionally, phytoestrogens abrogate autocrine and paracrine estrogen production by peritumoral stromal cells located in both primary and metastatic tumor sites. These phytoestrogens are common in legumes such as Glycyrrhiza glabra, G. uralensis (Licorice). It has also been reported that a compound isolated from Glycyrrhiza (isoliquirtigenin), displayed significant activity against aromatase in non-cellular, and in vivo assays (3.8 and 3 μM IC50, respectively).
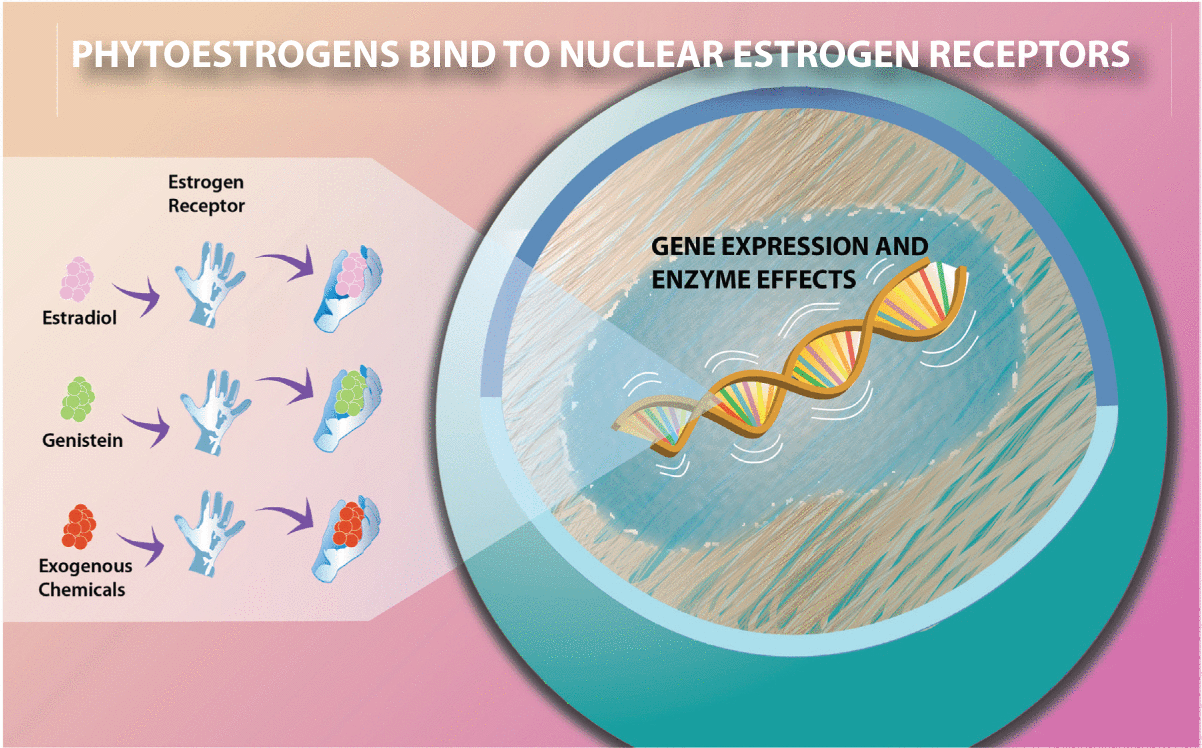
Estrogen enters cells, travels to the nuclear membrane, and binds to the membrane, eliciting effects on the nuclear DNA. Phytoestrogens can also bind nuclear estrogen receptors and have physiologic/medicinal effects.
PHYTOESTROGENS AS A TREATMENT FOR REPRODUCTIVE CANCERS
Phytoestrogens have only recently become a focus of attention in the treatment of reproductive cancers. The most effective treatment strategies and dosages of phytoestrogens are still undetermined. As recently as 10 years ago, the administration of phytoestrogens to cancer patients was highly controversial, owing to concerns over the possible proliferative effects that these natural estrogen derivatives might display. However, recent studies have shown that phytoestrogen supplementation is not associated with an increased risk of breast or endometrial cancer. In fact, selected phytoestrogens were associated with a favorable prognosis in progesterone receptor-positive breast cancer cells.
In 2012, the results of a human clinical trial on men (50-75 years of age) with elevated PSAs and negative prostate biopsies investigated the use of isoflavone (at a dose of 60 mg daily) on PSA and equol levels, as well as the progression to prostate cancer within a 12 month period. While there were no significant differences observed in PSA and equol levels between the clinical trial and placebo groups, a diagnosis of prostate cancer was significantly lower in the group receiving the isoflavone.
The above-described effects of phytoestrogen activity combined with the results of recent research suggest that phytoestrogen consumption may be beneficial for the prevention and treatment of breast and prostate cancers.
DISCLOSURE OF INTERESTS
Dr. Saunders reports personal fees related to employment or seeing patients from CCNM, the Dundas Naturopathic Centre, and from Beaumont Health Systems, Troy Hospital, MI, outside the submitted work. Dr. Winston reports personal fees from Herbalist & Alchemist, Inc, outside the submitted work. Dr. Stansbury has nothing to disclose.
REFERENCES
- Birnbaum, LS and Fenton, SE. Cancer and developmental exposure to endocrine disruptors.
- Ho, M-S, Tang, W-Y, de Frausto, JB, et.al. Developmental Exposure to Estradiol and Bisphenol A Increases Susceptibility to Prostate Carcinogenesis and Epigenetically Regulates Phosphodiesterase Type 4 Variant 4.
- Wu AH, Wan P, Hankin J, et.al. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans.
- Jakes RW, Duffy SW, Ng FC, et.al. Mammographic parenchymal patterns and self reported soy intake in Singapore Chinese women.
- Parkin DM, Bray F, Ferlay J, et.al. Global Cancer Statistics, 2002. CA
- Hwang YW, Kim SY, Jee SH, et.al. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies.
- Zhao E and Mu Qing. Phytoestrogen Biological Actions on Mammalian Reproductive System and Cancer Growth.
- Low YL, Dunning AM, Dowsett M, et.al. Phytoestrogen exposure is associated with circulating sex hormone levels in postmenopausal women and interact with ESR1 and NR1I2 gene variants.
- Subramanian A, Salhab M, Mokbel K. Oestrogen producing enzymes and mammary carcinogenesis: a review.
- Auricchio F, Migliaccio A, Castoria G. Sex-steroid hormones and EGF signalling in breast and prostate cancer cells: Targeting the association of Src with steroid receptors.
- Cassidy A, Bingham S, Setchell K. Biological effects of isoflavones in young women – importance of the chemical composition of soybean products.
- Li L, Andersen ME, Heber S, Zhang Q. Non-monotonic dose-response relationship in steroid hormone receptor-mediated gene expression.
- Payen L, Sparfel L, Courtois A, Vernhet L, Guillouzo A, Fardel O. The drug efflux pump MRP2: Regulation of expression in physiopathological situations and by endogenous and exogenous compounds.
- Bliedtner A, Zierau O, Albrecht S, Liebhaber S, Vollmer G. Effects of genistein and estrogen receptor subtype-specific agonists in ArKO mice following different administration routes.
- Hsieh T, Wu JM. Targeting CWR22R nu 1 prostate cancer cell proliferation and gene expression by combinations of the phytochemicals EGCG, genistein and quercetin.
- Doerge DR, Sheehan DM. Goitrogenic and estrogenic activity of soy isoflavones.
- Sosic-Jurjević B, Filipović B, Ajdzanović V, Savin S, Nestorović N, Milosević V, Sekulić M. Suppressive effects of genistein and daidzein on pituitary-thyroid axis in orchidectomized middle-aged rats.
- Son HY, Nishikawa A, Ikeda T, Imazawa T, Kimura S, Hirose M. Lack of effect of soy isoflavone on thyroid hyperplasia in rats receiving an iodine-deficient diet.
- Morani A, Warner M, Gustafsson J-. Biological functions and clinical implications of oestrogen receptors alfa and beta in epithelial tissues.
- Hermann RM, Wolff HA, Jarry H, Thelen P, Gruendker C, Rave-Fraenk M, et al. In vitro studies on the modification of low-dose hyper-radiosensitivity in prostate cancer cells by incubation with genistein and estradiol.
- Geller J, Sionit L, Partido C, et.al. Genistein inhibits the growth of human-patient BPH and prostate cancer in histoculture.
- Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, et al. Genistein mediated histone acetylation and demethylation activates tumor suppressor genes in prostate cancer cells.
- Kumar NB, Krischer JP, Allen K, Riccardi D, Besterman-Dahan K, Salup R, et al. Safety of purified isoflavones in men with clinically localized prostate cancer.
- Pendleton JM, Tan WW, Anai S, Chang M, Hou W, Shiverick KT, et al. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy.
- Jackson RL, Greiwe JS, Schwen RJ. Emerging evidence of the health benefits of S-equol, an estrogen receptor β agonist.
- Branca F, Lorenzetti S. Health effects of phytoestrogens.
- Fritz WA, Eltoum IE, Cotroneo MS, Lamartiniere CA. Genistein alters growth but is not toxic to the rat prostate.
- Strom SS, Yamamura Y, Duphorne CM, Spitz MR, Babaian RJ, Pillow PC, et al. Phytoestrogen intake and prostate cancer: A case-control study using a new database.
- Almstrup K, Fernandez MF, Petersen JH, Olea N, Skakkebaek NE, Leffers H. Dual effects of phytoestrogens result in U-shaped dose-response curves.
- Sasano H, Nagasaki S, Miki Y, Suzuki T. New Developments in Intracrinology of Human Breast Cancer Estrogen Sulfatase and Sulfotransferase.
- Frenette G, Leclerc P, D’Amours O, Sullivan R. Estrogen Sulfotransferase Is Highly Expressed Along the Bovine Epididymis and Is Secreted Into the Intraluminal Environment.
- Pasqualini JR. Estrogen Sulfotransferases in Breast and Endometrial Cancers.
- Day JM, Tutill HJ, Purohit A, Reed MJ. Design and validation of specific inhibitors of 17 beta-hydroxysteroid dehydrogenases for therapeutic application in breast and prostate cancer, and in endometriosis.
- Krazeisen A, Breitling R, Moller G, Adamski J. Phytoestrogens inhibit human 17 beta-hydroxysteroid dehydrogenase type 5.
- Le Bail JC, Champavier Y, Chulia AJ, Habrioux G. Effects of phytoestrogens on aromatase, 3 beta and 17 beta-hydroxysteroid dehydrogenase activities and human breast cancer cells.
- Spires TE, Fink BE, Kick EK, You D, Rizzo CA, Takenaka N, et al. Identification of novel functional inhibitors of 17 beta-hydroxysteroid dehydrogenase type III (17 beta-HSD3).
- Zhu YS, Sun GH. 5alpha-Reductase Isozymes in the Prostate.
- Cai LQ, Imperato-McGinley J, Zhu YS. Regulation of prostate 5-alpha-reductase-2 gene expression and prostate weight by dietary fat and caloric intake in the rat.
- Cai LQ, Cai J, Wu W, et.al. 17α-Estradiol and genistein inhibit high fat diet induced prostate gene expression and prostate growth in the rat.
- Drsata J. [Enzyme inhibition in the drug therapy of benign prostatic hyperplasia].
- Wadsworth TL, Worstell TR, Greenberg NM, Roselli CE. Effects of dietary saw palmetto on the prostate of transgenic adenocarcinoma of the mouse prostate model (TRAMP).
- Habib FK, Ross M, Ho CKH, Lyons V, Chapman K. Serenoa repens (Permixon (R)) inhibits the 5 alpha-reductase activity of human prostate cancer cell lines without interfering with PSA expression.
- Rhodes L, Primka RL, Berman C, Vergult G, Gabriel M, Pierre-Malice M, Gibelin B. Comparison of finasteride (proscar®), a 5α reductase inhibitor, and various commercial plant extracts in in vitro and in vivo 5α reductase inhibition.
- Pelletier G. Expression of steroidogenic enzymes and sex-steroid receptors in human prostate.
- Onsory K, Sobti RC, Al-Badran AI, Watanabe M, Shiraishi T, Krishan A, et al. Hormone receptor-related gene polymorphisms and prostate cancer risk in North Indian population.
- Balunas MJ, Kinghorn AD. Natural Compounds with Aromatase Inhibitory Activity: An Update.
- Musa MA, Cooperwood JS, Khan MOF. A Review of Coumarin Derivatives in Pharmacotherapy of Breast Cancer.
- Paoletta S, Steventon GB, Wildeboer D, Ehrman TM, Hylands PJ, Barlow DJ. Screening of herbal constituents for aromatase inhibitory activity.
- Tempfer CB, Froese G, Heinze G, Bentz E, Hefler LA, Huber JC. Side Effects of Phytoestrogens: A Meta-analysis of Randomized Trials.
- Roberts H. Safety of herbal medicinal products in women with breast cancer.