Abstract
Autoimmune diseases are a group of disorders in which the immune system dysfunctions and attacks host tissues. Although the pathogenesis of autoimmune thyroid disease has not been elucidated, there are several factors that have been associated with the disorder. Factors include genetic predisposition, nutrient deficiencies, use of certain medications affecting thyroid function, and environmental factors including exposure to radiation, heavy metals, and chemical contaminants. Thyroid disorders are often treated with drug therapy, which often have serious side effects and do not necessarily treat the underlying condition leading to the thyroid dysfunction. In recent years there has been increased interest in herbs and supplements as individuals take more interest in their health and well being. For autoimmune disease, vitamin D supplementation is recommended as deficiency in this nutrient has been associated with the disorder. Additionally, it also modulates T cell response and inhibits Th1 cytokines. In cases of autoimmune hyperthyroid disorder, rosmarinic acid, selenium and iodide supplementation are recommended. For autoimmune hypothyroid disorder, blue flag (Iris versicolor) and guggul (Commiphora mukul), selenium and iodide supplementation are indicated. Each of these supplements plays a specific role in restoring normal thyroid function. Further, rosmarinic acid found in plants such as rosemary (Rosmarinus officinalis), bugleweed (Lycopus virginicus), and lemon balm (Melissa officinalis) also calms excess T cell activity and pro inflammatory cytokine release. The use of these combinations of supplements should restore thyroid hormone homeostasis in autoimmune thyroid disorders. Proper medical supervision is required to ensure these herbs and nutrients are used safely and potential adverse effects are avoided.
CLINICAL PEARL PAPER:These are opinion articles written by clinicians who have had successful clinical experience. Although the protocols discussed are not derived from double-blind studies the articles offer clinicians valuable practical clinical skills that can be helpful in treating their patients effectively. |
INTRODUCTION
In a normal state, the immune system protects the body against foreign substances which can give rise to infection or disease. In response to foreign substances, the immune system produces antibodies that signal blood cells to destroy them. In the case of an autoimmune disorder, the system dysfunctions and the defense system attacks tissues and substances that are normally present in the body. The reasons behind the immune system no longer differentiating between healthy body tissues and antigens are not fully understood. Tissues and organs that are commonly affected by autoimmune disorders include blood vessels and blood cells, connective tissues, muscles and joints, and endocrine glands, with the most commonly affected organ being the thyroid.
The thyroid is touted as the most important gland in the body due to its ability to produce the hormones thyroxine (T4), triiodothyronine (T3) and calcitonin. Through the actions of thyroid peroxidase, iodine is covalently bound to tyrosine residues in the thyroglobulin molecules. These tyrosine molecules combine to form T4 and T3 on the thyroglobulin. Then lysosomal proteases sever the T4 and T3 from the thyroglobulin and the hormones are released into circulation. Thyroid hormones have several functions including regulating metabolism, directing cellular activity, stimulating heart muscles, nerves and brain functioning as well as increasing utilization of carbohydrates, proteins, fats and cholesterol. The thyroid produces several immunologic factors and has complex nutrient requirements in order to function properly and facilitate hormone synthesis. Thyroid hormone metabolism has a step up and step down metabolism where T4 can either be stepped up to the active form, T3, or stepped down to the inactive form, reverse T3 (rT3), both of which are further converted to 3,3′-T2.
The complexity of the thyroid makes it vulnerable to autoimmune disorders and dysfunction. Autoimmune thyroid disease occurs when the immune system attacks its own thyroid cells. This may result in reducing or even destroying the thyroid. Factors that may contribute to endocrine disease of the thyroid include viral infection, underlying disease, autoimmunity, congenital factors, gender, ethnicity, dietary iodine intake, and environmental factors among others.
PREVALENCE
Hypothyroidism, most often caused by autoimmune thyroiditis (Hashimoto thyroiditis) or iodine deficiency, is defined by a lack of thyroid hormones. In a recent systematic review, hypothyroidism has been reported to have an estimated incidence between 80/100,000/year in males and 350/100,000/year in females. Hyperthyroidism is characterized by an over activity of the gland resulting in elevated circulating free thyroid hormones. The incidence of hyperthyroidism is estimated to be between 8/100,000/year in males to 80/100,000/year in females. Prevalence of both types of thyroid disorders differ between men and women with women having higher incidence than men in both cases. The reason for the difference between genders is not clear. Studies conducted in various parts of the world suggest that the incidence of thyroid disease is changing, becoming more common than it was previously.
The Colorado Prevalence Study found that approximately 10% of subjects who were not taking any thyroid medications had an abnormality. Extrapolated to the United States adult population, this represents a possible 13 million cases of undetected thyroid gland abnormalities. These results suggest that physicians should consider monitoring TSH levels of all patients, especially in those who present with symptoms related to thyroid dysfunction.
PATHOGENESIS
There are two mechanisms that are responsible for autoimmune thyroid disease: 1) a T cell-mediated autoimmunity is seen in Hashimoto’s disease (chronic autoimmune thyroiditis and autoimmune hypothyroidism) and post-partum thyroiditis, and 2) a humoral response and presence of anti-thyroid stimulating hormone (TSH) receptor antibodies are seen in Graves’ disease. Both are characterized by T and B cell infiltration of the thyroid, abnormal thyroid function and production of thyroid autoantibodies.
B and T lymphocyte-mediated autoimmunity in Graves’ disease is known to be directed at four thyroid antigens that are key to thyroid function, namely, thyroglobulin (Tg), thyroid peroxidase (TPO), sodium-iodide symporter (NIS) and thyrotropin receptor (TSH-R). Each antigen serves a specific function. The sodium iodide symporter (NIS) transports iodide from blood into colloid tissue; thyroid peroxidase takes the iodide and attaches it to the tyrosine molecule producing T4; T4 is then converted to T3 in the peripheral tissues by iodothyronine deiodinase.
Thyroglobulin antibody is an antibody directed against thyroglobulin, and thyroid peroxidase antibody is directed against thyroid peroxidase, the enzyme responsible for the production of T4. Formation of serum thyroid peroxidase (TPO) and thyroglobulin antibodies (TgAb) are associated with all types of autoimmune thyroid disease and their presence in the blood can be used to diagnose underlying autoimmunity without the presence of clinical symptoms. More specifically, an elevation of these autoimmune antibodies may precede diagnosis of autoimmune disease diagnosis by 2 to 7 years.The measurement of thyroid stimulating hormone (thyrotropin) is a valuable measure to diagnose mildly elevated thyrotropin, subclinical hypothyroidism and hypothyroidism. Thyrotropin levels above 4.0 μIU/ml with TgAb or TPO positivity have been reported to predict future hypothyroidism in women.The presence of thyroglobulin antibody alone, is not a definitive indicator of thyroid disease although thyroglobulin antibodies are elevated in more than 90% of patients with Hashimoto (autoimmune) thyroiditis. Elevation of thyroglobulin antibody has been seen in patients with other autoimmune disorders including patients with systemic lupus erythematosus. Elevation of both thyroglobulin antibody and thyroid peroxidase antibody have been observed in patients with autoimmune thyroiditis, rheumatoid arthritis, pernicious anemia and type I diabetes. Additionally, the presence of thyroglobulin antibody, but not thyroid peroxidase has been associated with thyroid cancer.
ENVIRONMENTAL FACTORS
Autoimmune thyroiditis is mediated by lymphocytes and cytokines which destroy the thyroid gland. Thyroid antigens are influenced by a host of environmental factors including toxins, halogens, pesticides, heavy metals and nutrient deficiencies among others. Although it is reported that there is a genetic predisposition, onset of the disease can be triggered by environmental factors including iodine intake and the proliferation of lymphocytes. The production of antibodies is stimulated by the iodination of thyroglobulin.
HEAVY METALS:
Heavy metals (cadmium, lead and mercury) have been linked to alterations in thyroid function. The association of mercury with thyroglobulin antibody positivity, but not thyroid peroxidase antibody positivity, indicates a relationship between mercury and autoimmunity. Preclinical evidence suggests that thyroid peroxidase enzyme is inhibited by inorganic mercury but not organic mercury. Both organic and inorganic mercury inhibit the iodination of thyroglobulin. Further, it has been hypothesized that mercury induces protein alterations which result in cell-specific antigenicity as well as a theory that formation of polyclonal B lymphocytes and autoantibodies result from mercury induced T lymphocyte stimulation. Mercury exposure typically reflects consumption of fish in the general population. Cadmium exposure leads to lipid peroxidation in the thyroid, which can be protected against by ascorbic acid supplementation. Alterations in antioxidant enzyme systems and lipid peroxidation in response to heavy metals are hypothesized to lead to membrane integrity dysfunction and suboptimal 5′-deiodinase activity. In order for the body to remove cadmium, selenium becomes bound to the cadmium and is expelled from the body via the bile system. Selenium is an essential component of deiodinase enzymes responsible for conversion of T4 to T3, and reduced selenium also leads to an increase in rT3 which in turn can result in hypothyroidism.Selenium is also an essential component of the antioxidant, glutathione peroxidase. Reduction in selenium reduces the formation of glutathione peroxidase, resulting in increased levels of reactive oxygen species and hydrogen peroxide which can damage the thyroid.
HALOGENS AND PESTICIDES:
Iodination of thyroglobulin and the sodium iodide symporter are affected by halogens including perchlorate, chlorine, fluorine and bromine. Each of these chemicals have differing effects on the thyroid and vary in their mechanisms of action. Perchlorate (ClO4) competitively inhibits iodide uptake by the NIS and displaces T4 from serum. Chlorine inhibits iodide trapping and decreases serum T4. Fluoride decreases T3 and T4 and increases TSH in the blood; however, the mechanism by which this occurs remains unclear. Bromine displaces iodine, increases plasma TSH and has an inhibitory effect on thyroid activity. These individual chemicals are found in our environment and often in household products including beverages, drinking water, toothpastes and cleaning agents; however, adverse effects on the thyroid are generally seen only with exposure to high amounts of these compounds. Organochlorine pesticides, which contain the chlorine molecule, have also been associated with increased incidence of euthyroid goiter or hypothyroidism. Usually the thyroid hormone concentration change occurs in response to a mixture of chemicals, but not to individual chemicals.
POLYHALOGENATED BIPHENYLS:
Polyhalogenated biphenyls such as polybrominated biphenyls and polychlorinated biphenyls are commonly used compounds with many industrial applications including flame retardants, foams, building materials, lubricants, adhesives, inks and plasticizers. They have been known to accumulate in water ways and are incorporated into the adipose tissue of fish and humans. Toxins such as polychlorinated biphenyls (PCBs), polybrominated diphenyl ether (PBDE) and bisphenyl A (BPA) have been linked to modulation of thyroid hormones. PCBs resemble thyroid hormone structurally and have been shown to disrupt thyroid function in vivo. Blood concentrations of PCBs negatively correlate with circulating thyroid hormone levels.Immunomodulatory effects of PCBs may be responsible for increases in thyroid peroxidase antibodies and subsequent interference with iodide transport seen in populations with long-term exposure to PCBs. One study has reported that as PBDE increases, increased sub-clinical hyperthyroidism was seen in pregnant women. The structure of PBDE is similar to thyroid hormone and can displace T4 from serum thyroid binding protein transthyretin (TTR). BPA, commonly found in plastic and metal food packaging, is believed to disrupt T3 signaling pathways. It has been suggested that BPA may displace T3 from thyroid receptors and result in gene suppression.
ADDITIONAL FACTORS
Other factors that can lead to thyroid dysfunction include radiation, infections, medications, and nutritional deficiencies. Heavy industry pollutants, coal pollution, motor vehicle emissions and agricultural fungicides have also been suggested to play a role in autoimmune thyroid disease. Medications that have reported effects on thyroid antigens include beta blockers, theophyline, amiodarone, phenytoin, propylthiouracil (PTU) and chemotherapy. Nutritional deficiencies in selenium, iodine, iron, zinc, vitamin A and vitamin B12 have been associated with hypothyroidism. As an example, vitamin B12 deficiency results in decreased levels of 5′-deiodinase, which is utilized in the metabolism of T4 to T3 in peripheral tissues.
STRESS AND THYROID HORMONE METABOLISM
Stressors are endogenous or exogenous forces that challenge the homeostasis of an organism. When this homeostasis is challenged or a threat is perceived, the stress response is activated. The stress response is mediated in the central nervous system as well as peripheral organs. The typical response to stress is an increase in production of cortisol, decrease in production of TSH, decrease in T3 and increase in rT3. Altered peripheral metabolism of T4 to T3 and rT3 may be due to increased cortisol. Studies have shown that regardless of the source of stress, rT3 increases in response and the time to return to normal hormone metabolism may vary. The mechanism by which thyroid metabolism is influenced may vary by type of stressor. Intense exercise, lack of sleep, and caloric restriction are some of the various stressors that have been reported in the literature to affect cortisol levels and/or peripheral thyroid hormone metabolism, with the latter resulting in an increase of rT3 at the expense of T3.
CALORIE RESTRICTION AND FASTING:
Proper nutrition is important for many aspects of health and well-being. Proper function of the thyroid and thyroid hormone metabolism are no exception. It has been established that nutrition and caloric restriction impact thyroid metabolism centrally and possibly peripherally. In the case of caloric restriction, T3 levels decrease and rT3 increase suggesting a modulation of hepatic 5′-deiodinase activity. However, the effects of caloric restriction on thyroid hormone metabolism appear to reverse, returning to a normal balance, after three continuous weeks of dieting. Several factors may influence the response during caloric restriction including macronutrient content of the diet, genetics, weight and gender.
Fasting also impacts thyroid hormone metabolism, possibly due to elevated cortisol levels. Studies suggests that the modulation may be due to influences on central as well as peripheral metabolism. Similar to the typical response to stress, fasting serum rT3 levels increase and T3 levels decrease compared to the non-fasting state. Prolonged periods of fasting or caloric deprivation result in a similar thyroid hormone profile.
Changes in thyroid metabolism are not restricted to caloric deprivation. Increased caloric intake reportedly results in an increased clearance of rT3; however, the effects on other pathways have not been studied. Changes in thyroid hormone levels appear to be associated with energy expenditure relative to caloric intake. As such, the balance between caloric intake and energy expenditure is key to maintaining normal thyroid hormone metabolism. Certain types of foods, such as cruciferous vegetables, may also affect the thyroid. Hypothyroidism has been reported in a patient following chronic ingestion of very high amounts of bok choy. This association has also been demonstrated in animals. However, cooked cruciferous vegetables do not seem to have an effect on the thyroid. Consumption of 150 g/day (5 oz/day) of cooked Brussels sprouts for 4 weeks had no adverse effects on thyroid function. The mechanism involved is not clear, but it is possible that the glucosinolates found in cruciferous vegetables may yield goitrin, a compound known to interfere with thyroid hormone synthesis. Another possible explanation is the release of thiocyanate ions due to hydrolysis of glucosinolates. Thiocyanate ions compete with iodine for uptake by the thyroid, which can result in hypothyroidism in patients with iodine deficiency. Myrosinase, an enzyme present in raw brassica, accelerates glucosinolate hydrolysis. During cooking, this enzyme is deactivated and may explain the varying results reported between raw and cooked cruciferous vegetables.
SLEEP DEPRIVATION:
Both central and peripheral thyroid metabolism appears to be influenced by short-term sleep deprivation. After a night of sleep deprivation, T4, T3 and rT3 increase. The effects of long-term sleep deprivation have not yet been investigated.
ALCOHOL CONSUMPTION:
A decrease in T3 and T4 and increase in rT3 has been reported in chronic alcohol consumers, which may be due to the impairment of 5′-deiodinase in the liver by alcohol or increased cortisol. The effects of moderate alcohol consumption on thyroid hormone metabolism has not yet been investigated and warrants consideration.
EXERCISE:
The effects of exercise on modulation of thyroid hormone metabolism are not completely clear as the response seems to be influenced by the level of conditioning of subjects prior to exposure to intense exercise. Exercise appears to influence T3 and rT3 but not T4. The mechanism has not yet been identified, but may be due to increased levels of cortisol during and after exercise. Future research is warranted to understand the effects of varying levels of exercise (e.g. moderate vs. intense), type of exercise (e.g. resistance vs. aerobic) and condition of subjects prior to exposure to this type of stress.
Studies have also found that exercise-related energy expenditure exceeding calories consumed may result in low T3 and increased rT3. It is believed that in this scenario peripheral metabolism of T4 and decreased hepatic 5′-deiodinase activity is likely. Such an occurrence may be overcome by consuming more calories without changing exercise intensity or duration.
HERBS AND NUTRIENTS FOR AUTOIMMUNE THYROID DISORDERS
Thyroid disorders are often treated with drug therapy. In the case of Hashimoto’s disease, the standard medical protocol is thyroid hormone replacement. For Graves’ disease, anti-thyroid drugs, operations to partially or fully remove the thyroid gland and use of radioactive iodine (131I) are standard recommendations of physicians. Due to potential side effects associated with many medications some people seek complementary and alternative therapies. Others may seek alternative therapies due to their cultural backgrounds. The 2002 Health and Diet Survey sponsored by the US FDA found that 73% of adults aged 18 years or older used a dietary supplement in the previous 12 months. Some commonly used natural products for autoimmune thyroid disorders include rosmarinic acid, blue flag, guggul, selenium, iodide and vitamin D3 supplements.
ROSMARINIC ACID:
Rosmarinic acid is found in several members of the Lamiaceae family plants such as rosemary (Rosmarinus officinalis), bugleweed (Lycopus virginicus), and lemon balm (Melissa officinalis) and has medicinal uses in several cultures. Its physiological functions and medicinal properties are well characterized. Based on in vivo research, rosmarinic acid is hypothesized to prevent TSH effects on receptor sites, block immunoglobulin effects on TSH receptors, and inhibit peripheral conversion of thyroxine to T3. These findings suggest that rosmarinic acid may be beneficial in Graves’ disease.
Rosmarinic acid has been shown to be capable of inducing T cell apoptosis of only actively proliferating T cells,direct T cell activity, inhibit T cell activation and proliferation and modulate T cell promotion of pro-inflammatory cytokine release, suggesting potential application in autoimmune disorders.
BLUE FLAG (IRIS VERSICOLOR):
Blue flag was traditionally used by American natives for its medicinal effects and is an adaptogenic herb. Adding adaptogenic herbs is often helpful in the treatment of Hashimoto’s disease because they act as endocrine tonics that help mitigate autoimmune diseases. It is used as an alternative therapy due to its reported lymphatic anti-inflammatory effects, and has been used in liver and spleen enlargement (hepato-splenomegaly) and thyroid enlargement (thyromegaly). Although clinical research is lacking, blue flag has a long history of medicinal use for treatment of goiters and thyroid enlargement.
GUGGUL (COMMIPHORA MUKUL):
Guggul is an extract of the oleo-resin of the Commiphora mukul tree, native to India. Guggul has demonstrated biological effects on thyroid homeostasis and lipid lowering properties. Guggul is used as a thyroid stimulant in traditional Indian Ayurvedic medicine. It has been shown to improve both thyroid function and structure in melatonin induced hypothyroidism in mice. Studies have identified a ketosteroid present in the oleo-resin of C. mukul (Z-guggulsterone) which has strong thyroid stimulatory activity. In vivo, administration of Z-guggulsterone increased iodine-uptake by the thyroid and increased activities of both thyroid peroxidase and protease. Guggulsterone also seems to increase T3 synthesis by increasing the conversion of T4 to T3 and significantly decrease hepatic lipid peroxidation. Since serum thyroxine (T4) is converted to T3 in the liver, researchers concluded that hormone levels and peroxidation are related. It is believed that Guggul’s effect on thyroid regulation is responsible for the therapeutic effects seen in cholesterol levels.
SELENIUM:
Selenium, a constituent of selenoproteins, has been implicated in the autoimmune thyroiditis by increasing the duration and exacerbating disease severity. It is possible that this is due to reduced activity of the selenoprotein glutathione peroxidase leading to increases in hydrogen peroxide production. Iodothyronine selenodeiodinases D1 and D2, are another class of selenoproteins which produce active T3 through deiodination in peripheral tissues.
During selenium supplementation serum selenium has been reported to increase by 45%, plasma glutathione peroxidase by 21% and TPO antibody decreased by 76%. On withdrawal of supplementation, a sharp decrease was seen in selenium and glutathione peroxidase accompanied by marked increase in TPO. Extracellular glutathione peroxidase is secreted by thyrocytes and primarily modulates hydrogen peroxidase and organification of iodine. Its secondary function is to prevent oxidation damage to the thyrocytes themselves.
IODINE:
Potassium iodide and T4 therapy are often used for treating thyroid disorders as they have been shown to inhibit and prevent the growth of benign thyroid nodules in 66% of patients. However, caution must be used when prescribing iodine in the presence of a hot nodule. Ingested iodide is organified to iodine and bound to thyroglobulin in the follicle by thyroid peroxidase and hydrogen peroxide. Premature organification of iodine in the follicular cell is prevented by glutathione peroxidase. Low levels of glutathione peroxidase in the thyroid tissue can lead to damage to the NIS. High doses of iodide can lead to an over saturation of the NIS and lead to iodothyronine suppression.
Iodine is a crucial constituent of thyroid function and is a component of thyroxine and T3. It is well established that the highest prevalence of thyroid disorders is seen in populations where iodine deficiency is prevalent. Autoimmune hypothyroidism is not commonly seen in mild to moderate iodine deficiency. However, thyrogloblin antibody is more common in iodine deficiency, suggesting that thyroid globulin antibodies are generated in response to thyroid globulin release from an iodine deficient thyroid. Goiter is usually endemic in areas where daily iodine intake is <50 mg and congenital hypothyroidism is seen in areas where intake is <25 mg/day. Approximately 30% of the world’s population live in areas of iodine deficiency. These areas see a prevalence of goiter as high as 80%. Iodine supplementation has been reported to reduce thyroid volume with sustained effects seen through 12 months post-therapy in patients with goiter. In contrast, though T4 therapy also decreases thyroid volume, thyroid volume increases when therapy is removed. Iodine is also reported to reduce thyroglobulin concentrations while maintaining stable levels of serum TSH. In areas of iodine repletion, those with thyroid dysfunction have a high prevalence of autoimmune disease. These autoimmune diseases range from Hashimoto’s disease to thyrotoxicosis resulting from Grave’s disease.
VITAMIN D3:
In individuals with Hashimoto’s disease, circulating vitamin D3 levels are low, similarly, vitamin D deficiency has been associated with other autoimmune conditions. Animal models have demonstrated that vitamin D3 supplementation has been shown to prevent the development of autoimmune conditions including autoimmune thyroiditis. Vitamin D plays a role in immunity, with low intake resulting in enhanced immune response and high intake resulting in suppression of the immune response. Vitamin D3 modulates T cell responses, inhibiting Th1 cell cytokines including IFN-g and IL-2. Th1 cell activation and production of cytokines is necessary for cell-mediated immune responses, which, in autoimmune diseases is misdirected. Although there are no clinical studies in which vitamin D supplementation alone has been used as a treatment for autoimmune thyroid disease, supplementation is recommended due to the association of vitamin D deficiency with autoimmune disorders. Blood levels of vitamin D3 should be monitored when treating patients with vitamin D3 to ensure sufficiency is achieved (50–70 nM) and levels associated with hypercalciuria (250 nM) are not reached.
SUGGESTED PROTOCOL:
Due to the dearth of clinical evidence, practitioners often use natural ingredients in practice on the basis of traditional or historical use and fundamental evidence presented in in vitro and in vivo scientific literature and individual case studies. This article presents a review of the body of in vitro and in vivo research on autoimmune thyroid disease and suggests a protocol of supplements that may benefit patients with autoimmune thyroid disease. These recommendations are based on the varying effects that these natural health ingredients have on thyroid metabolism and immune modulation
SUPPLEMENTS:
Vitamin D 50,000 up to two times per week is suggested in addition to stress reduction, healthy digestion and absorption, proper nutrition and exercise. A recommended supplementation protocol for hypothyroidism is Iris (Blue Flag) 750 mg per day and Guggul 750 mg per day for their anti-inflammatory effects, and for thyroid support, iodine 6–24 mg per day and selenium 400 μg per day. For hyperthyroid, it is recommended that patients take rosmarinic acid 335 mg twice per day, iodine 6 mg per day and selenium 400 μg per day (Table 1). These herbs and nutrients should be used under medical supervision to ensure their safe and effective use. Theoretically, overuse or abuse of blue iris can increase the risk of adverse effects from cardiac glycoside drugs such as digoxin (Lanoxin) and therefore should not be taken concomitantly. Concomitant use of large amounts of guggulsterones might increase the adverse effects of hormone replacement therapy through estrogen-alpha receptor agonist activity and should not be taken with estrogens. Mild and transient gastrointestinal side effects may occur when first using some of these supplements and include nausea, bloating and loose stools/diarrhea. These side effects have generally been reported with higher dosages of these supplements.
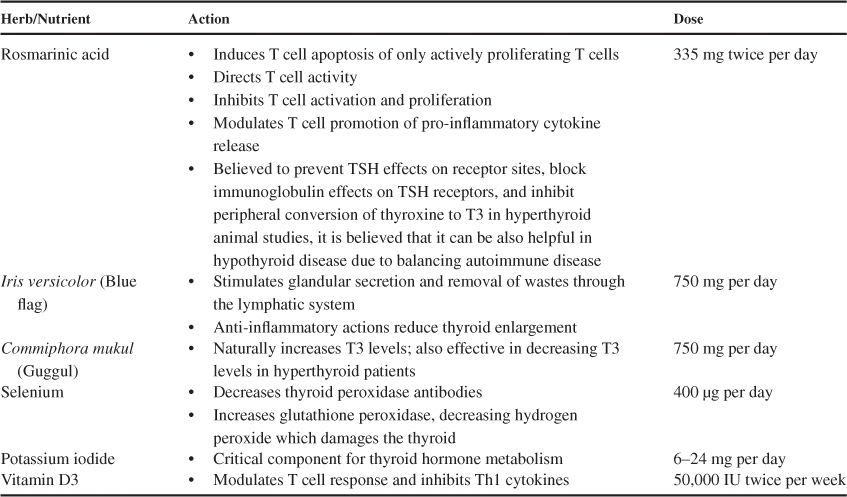
Summary of key herbs and nutrients, their mechanisms of action and recommended doses for autoimmune thyroid disorders.
CONCLUSION
Supplemental vitamin D3, rosmarinic acid, guggul, blue flag, selenium and iodine may be of benefit to patients with autoimmune thyroid disorders based on the body of in vitro and in vivo evidence reviewed. Each of these compounds have varied mechanisms of action supporting thyroid hormone homeostasis. Vitamin D3 is indicated in autoimmune diseases as deficiency in this vitamin has been associated not only with autoimmune thryoiditis, but also with several other autoimmune disorders (including rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease), thus achieving sufficient levels of vitamin D3 in the bloodstream is important. Further, vitamin D3 also plays a role in T cell response and inhibition of Th1 cytokines. In addition to supporting thyroid function, rosmarinic acid also plays a role in T cell modulation and response of pro-inflammatory cytokines, suppressing the immune response, which in turn prevents damage to tissues and organs such as the thyroid. In combination with herbs and nutrients and specific protocols suggested, complementary therapies are recommended including stress reduction, ensuring healthy digestion and absorption, and proper nutrition, low in foods that can interfere with thyroid function (i.e. cruciferous vegetables). Exercise is equally important as proper nutrition, however it is imperative that energy expenditure does not exceed caloric intake. These supplements should be used under medical supervision and physicians need to be cognizant of potential side effects and contraindications with medications such as digoxin and estrogens.
DISCLOSURE OF INTERESTS
Dr. Friedman reports receipt of salary from WTSMED, outside the submitted work.
REFERENCES
- McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity.
- Ishii H, Inada M, Tanaka K, et al. Sequential deiodination of thyroxine in human thyroid gland.
- McGrogan A, Seaman HE, Wright JW, de Vries CS. The incidence of autoimmune thyroid disease: a systematic review of the literature.
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study.
- Spitzweg C, Joba W, Heufelder AE. Expression of thyroid-related genes in human thymus.
- Hutfless S, Matos P, Talor MV, Caturegli P, Rose NR. Significance of prediagnostic thyroid antibodies in women with autoimmune thyroid disease.
- Baskin HJ, Cobin RH, Duick DS, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism.
- Walsh JP, Brenner AP, Feddema P, Leedman PJ, Brown SJ, O’Leary P. Thyrotropin and thyroid antibodies as predictors of hypothyroidism: a 13-year, longitudinal study of a community-based cohort using current immunoassay techniques.
- Gallagher CM, Meliker JR. Mercury and thyroid autoantibodies in U.S. women, NHANES 2007–2008.
- Kim ES, Lim DJ, Baek KH, et al. Thyroglobulin antibody is associated with increased cancer risk in thyroid nodules.
- Gupta P, Kar A. Role of ascorbic acid in cadmium-induced thyroid dysfunction and lipid peroxidation.
- Swarup D, Naresh R, Varshney VP, et al. Changes in plasma hormones profile and liver function in cows naturally exposed to lead and cadmium around different industrial areas.
- Wade MG, Parent S, Finnson KW, et al. Thyroid toxicity due to subchronic exposure to a complex mixture of 16 organochlorines, lead, and cadmium.
- Yoshizuka M, Mori N, Hamasaki K, et al. Cadmium toxicity in the thyroid gland of pregnant rats.
- Nishida M, Sato K, Kawada J. Differential effects of methylmercuric chloride and mercuric chloride on oxidation and iodination reactions catalyzed by thyroid peroxidase.
- Kawada J, Nishida M, Yoshimura Y, Mitani K. Effects of organic and inorganic mercurials on thyroidal functions.
- Pusey CD, Bowman C, Morgan A, Weetman AP, Hartley B, Lockwood CM. Kinetics and pathogenicity of autoantibodies induced by mercuric chloride in the brown Norway rat.
- Powell JJ, Van de Water J, Gershwin ME. Evidence for the role of environmental agents in the initiation or progression of autoimmune conditions.
- Agency for Toxic Substances and Disease Registry (ASTDR).
- Chaurasia SS, Kar A. Influence of lead on type-I iodothyronine 5′-monodeiodinase activity in male mouse.
- Chaurasia SS, Kar A. Protective effects of vitamin E against lead-induced deterioration of membrane associated type-I iodothyronine 5′-monodeiodinase (5′D-I) activity in male mice.
- Gupta P, Kar A. Cadmium induced thyroid dysfunction in chicken: hepatic type I iodothyronine 5′-monodeiodinase activity and role of lipid peroxidation.
- Yoshida K, Sugihira N, Suzuki M, et al. Effect of cadmium on T4 outer ring monodeiodination by rat liver.
- Merrill EA, Clewell RA, Gearhart JM, et al. PBPK predictions of perchlorate distribution and its effect on thyroid uptake of radioiodide in the male rat.
- Harrington RM, Shertzer HG, Bercz JP. Effects of chlorine dioxide on thyroid function in the African green monkey and the rat.
- Wang H, Yang Z, Zhou B, Gao H, Yan X, Wang J. Fluoride-induced thyroid dysfunction in rats: roles of dietary protein and calcium level.
- Allain P, Berre S, Krari N, et al. Bromine and thyroid hormone activity.
- Howdeshell KL. A model of the development of the brain as a construct of the thyroid system.
- Porter WP, Jaeger JW, Carlson IH. Endocrine, immune, and behavioral effects of aldicarb (carbamate), atrazine (triazine) and nitrate (fertilizer) mixtures at groundwater concentrations.
- Brouwer A, Morse DC, Lans MC, et al. Interactions of persistent environmental organohalogens with the thyroid hormone system: mechanisms and possible consequences for animal and human health.
- Hagmar L, Rylander L, Dyremark E, Klasson-Wehler E, Erfurth EM. Plasma concentrations of persistent organochlorines in relation to thyrotropin and thyroid hormone levels in women.
- Persky V, Turyk M, Anderson HA, et al. The effects of PCB exposure and fish consumption on endogenous hormones.
- Chevrier J, Harley KG, Bradman A, Gharbi M, Sjodin A, Eskenazi B. Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy.
- Marsh AB, Bergman A, Bladh LG, Gillner M, Jakobsson E. Synthesis of p-hydroxybromodiphenyl ethers and binding to the thyroid receptor.
- Moriyama K, Tagami T, Akamizu T, et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist.
- Mangano JJ. A post-Chernobyl rise in thyroid cancer in Connecticut, USA.
- Kronenberg HM, Polonsky KS, Melmed S, editors.
- Dong BJ. How medications affect thyroid function.
- Delange F. The disorders induced by iodine deficiency.
- Pizzulli A, Ranjbar A. Selenium deficiency and hypothyroidism: a new etiology in the differential diagnosis of hypothyroidism in children.
- Watts DL. The nutritional relationships of the thyroid.
- Duntas LH. Environmental factors and autoimmune thyroiditis.
- Safran M, Paul TL, Roti E, Braverman LE. Environmental factors affecting autoimmune thyroid disease.
- Stangl GI, Schwarz FJ, Kirchgessner M. Cobalt deficiency effects on trace elements, hormones and enzymes involved in energy metabolism of cattle.
- Chrousos GP. Stress and disorders of the stress system.
- Kelly GS. Peripheral metabolism of thyroid hormones: a review.
- Bugaresti JM, Tator CH, Silverberg JD, et al. Changes in thyroid hormones, thyroid stimulating hormone and cortisol in acute spinal cord injury.
- Azizi F, Amini M, Arbab P. Time course of changes in free thyroid indices, rT3, TSH, cortisol and ACTH following exposure to sulfur mustard.
- McCormack PD, Thomas J, Malik M, Staschen CM. Cold stress, reverse T3 and lymphocyte function.
- Johansson G, Laakso ML, Karonen SL, Peder M. Examination stress affects plasma levels of TSH and thyroid hormones differently in females and males.
- Limanova Z, Sonka J, Kratochvil O, Sonka K, Kanka J, Sprynarova S. Effects of exercise on serum cortisol and thyroid hormones.
- Radomski MW, Hart LE, Goodman JM, Plyley MJ. Aerobic fitness and hormonal responses to prolonged sleep deprivation and sustained mental work.
- Suda AK, Pittman CS, Shimizu T, Chambers JB Jr. The production and metabolism of 3,5,3′-triiodothyronine and 3,3′,5-triiodothyronine in normal and fasting subjects.
- O’Brian JT, Bybee DE, Burman KD, et al. Thyroid hormone homeostasis in states of relative caloric deprivation.
- Hugues JN, Burger AG, Pekary AE, Hershman JM. Rapid adaptations of serum thyrotrophin, triiodothyronine and reverse triiodothyronine levels to short-term starvation and refeeding.
- Palmblad J, Levi L, Burger A, et al. Effects of total energy withdrawal (fasting) on thelevels of growth hormone, thyrotropin, cortisol, adrenaline, noradrenaline, T4, T3, and rT3 in healthy males.
- Scriba PC, Bauer M, Emmert D, et al. Effects of obesity, total fasting and re-alimentation on L-thyroxine (T4), 3,5,3′-L-triiodothyronine (T3), 3,3′,5′-L-triiodothyronine (rT3), thyroxine binding globulin (TBG), cortisol, thyrotrophin, cortisol binding globulin (CBG), transferrin, alpha 2-haptoglobin and complement C′3 in serum.
- Burger AG, O’Connell M, Scheidegger K, Woo R, Danforth E Jr. Monodeiodination of triiodothyronine and reverse triiodothyronine during low and high calorie diets.
- Liang H, Juge-Aubry CE, O’Connell M, Burger AG. Organ-specific effects of 3,5,3′-triiodothyroacetic acid in rats.
- Chu M, Seltzer TF. Myxedema coma induced by ingestion of raw bok choy.
- Fenwick GR, Heaney RK, Mullin WJ. Glucosinolates and their breakdown products in food and food plants.
- McMillan M, Spinks EA, Fenwick GR. Preliminary observations on the effect of dietary brussels sprouts on thyroid function.
- Higdon J.
- Dekker M, Verkerk R, Jongen WM. Predictive modelling of health aspects in the food production chain: a case study on glucosinolates in cabbage.
- Keck E, Degner FL, Schlaghecke R. Alcohol and endocrinologic homeostasis.
- Langer P, Foldes O, Gschendtova K. Effect of ethanol and linoleic acid on changes in biliary excretion of iodothyronines possibly related to thyroxine deiodination in rat liver.
- Loucks AB, Callister R. Induction and prevention of low-T3 syndrome in exercising women.
- Loucks AB, Heath EM. Induction of low-T3 syndrome in exercising women occurs at a threshold of energy availability.
- Timbo BB, Ross MP, McCarthy PV, Lin CJ. Dietary supplements in a national survey: prevalence of use and reports of adverse events.
- Sourgens H, Winterhoff H, Gumbinger HG, Kemper FH. Effects of Lithospermum officinale and related plants on hypophyseal and thyroid hormones in the rat.
- Hur YG, Yun Y, Won J. Rosmarinic acid induces p56lck-dependent apoptosis in Jurkat and peripheral T cells via mitochondrial pathway independent from Fas/Fas ligand interaction.
- Hur YG, Suh CH, Kim S, Won J. Rosmarinic acid induces apoptosis of activated T cells from rheumatoid arthritis patients via mitochondrial pathway.
- Park SH, Oh HS, Kang MA, et al. The structure-activity relationship of the series of non-peptide small antagonists for p56lck SH2 domain.
- Won J, Hur YG, Hur EM, et al. Rosmarinic acid inhibits TCR-induced T cell activation and proliferation in an Lck-dependent manner.
- Kang MA, Yun SY, Won J. Rosmarinic acid inhibits Ca2+-dependent pathways of T-cell antigen receptor-mediated signaling by inhibiting the PLC-gamma 1 and Itk activity.
- Frances D. Botanical approaches to hypothyroidism: avoiding supplemental thyroid hormone.
- Singh AK, Tripathi SN, Prasad GC. Response of commiphora mukul (guggulu) on melatonin induced hypothyroidism.
- Tripathi YB, Malhotra OP, Tripathi SN. Thyroid stimulating action of Z-guggulsterone obtained from Commiphora mukul.
- Tripathi YB, Tripathi P, Malhotra OP, Tripathi SN. Thyroid stimulatory action of (Z)-guggulsterone: mechanism of action.
- Antonio J, Colker CM, Torina GC, Shi Q, Brink W, Kaiman D. Effects of a standardized guggulsterone phosphate supplement on body composition in overweight adults: a pilot study.
- Panda S, Kar A. Gugulu (Commiphora mukul) induces triiodothyronine production: possible involvement of lipid peroxidation.
- Zagrodzki P, Ratajczak R. Selenium supplementation in autoimmune thyroiditis female patient—effects on thyroid and ovarian functions (case study).
- Duntas LH. The role of selenium in thyroid autoimmunity and cancer.
- Ekholm R, Bjorkman U. Glutathione peroxidase degrades intracellular hydrogen peroxide and thereby inhibits intracellular protein iodination in thyroid epithelium.
- Grineva EN, Malakhova TV, Tsoi UA, Smirnov BI. [Efficacy of thyroxine and potassium iodide treatment of benign nodular thyroid lesions].
- Goodman HM.
- Kogai T, Liu YY, Richter LL, Mody K, Kagechika H, Brent GA. Retinoic acid induces expression of the thyroid hormone transporter, monocarboxylate transporter 8 (Mct8).
- Vanderpump MP. The epidemiology of thyroid disease.
- Kahaly GJ, Dienes HP, Beyer J, Hommel G. Iodide induces thyroid autoimmunity in patients with endemic goitre: a randomised, double-blind, placebo-controlled trial.
- Tamer G, Arik S, Tamer I, Coksert D. Relative vitamin D insufficiency in Hashimoto’s thyroiditis.
- Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence.
- Ginanjar E, Sumariyono,Setiati S, Setiyohadi B. Vitamin D and autoimmune disease.
- Chen W, Lin H, Wang M. Immune intervention effects on the induction of experimental autoimmune thyroiditis.
- Fournier C, Gepner P, Sadouk M, Charreire J. In vivo beneficial effects of cyclosporin A and 1,25-dihydroxyvitamin D3 on the induction of experimental autoimmune thyroiditis.
- Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions.
- Kivity S, Agmon-Levin N, Zisappl M, et al. Vitamin D and autoimmune thyroid diseases.